Sulfur, in tremendous abundance as byproducts of petroleum industry, is one of the most intriguing solutions to address the energy dilemma by manifesting the chemistry between sulfur and lithium. Thus, lithium-sulfur batteries employing lithium-sulfur redox couple theoretically deliver energy density of 2600 Wh kg-1, which is 3-5 times higher than traditional lithium-ion batteries. Although a promising prospect, there are still several obstacles hindering their practical application. One of the most significant is the rapid capacity fading.
"The fast capacity decay of the lithium-sulfur battery is ascribed to many aspects. One of the most widely accepted reasons is due to the intermediate polysulfides. Polysulfides are a transition form of sulfur, partially lithiated, which is highly polar and soluble in the typical organic electrolyte we used. During discharge, they dissolve in the electrolyte, diffuse from cathode to anode, and react with lithium anode. The active materials are lost in this way, causing capacity fading," said Dr. Qiang Zhang, an associate professor at Department of Chemical Engineering, Tsinghua University. "This issue causes enormous concern and considerable endeavor is dedicated to address this problem. But we are also interested in is another issue, the dynamic fluctuation of affinity between different sulfur species and conductive host materials."
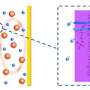
"Because of the multi-electron-transfer process, sulfur species vary from initial elemental sulfur, intermediate polysulfides, and final discharge product of lithium sulfides. Sulfur is unpolar, and thus exhibits highest affinity to conventional carbon hosts. While polsulfides and lithium sulfides are highly polar, weakening the interaction between them and carbon. Due to this poor interaction, they easily detach from the carbon host and contribute no capacity. As a result, the performance of a lithium-sulfur battery deteriorates rapidly when only pure carbon hosts are employed," said Qiang. "Consequently, a key issue is how to choose an ideal host material with high affinity to both unpolar sulfur and polar polysulfides, as well as lithium sulfides."
Herein, nitrogen-doped carbon nanotubes were adopted as host material for sulfur cathode. Nitrogen atoms with higher electronegativity are incorporated into the graphitic lattices of carbon nanotubes, which has been demonstrated capability to tune the electronic structure and surface properties. How does the doping nitrogen atoms affect the electrochemical behavior when nitrogen-doped carbon nanotubes are applied for lithium-sulfur battery?
Hong-Jie Peng, a graduate student and the first author, deliberately answered this question. "Firstly, we conducted a density functional theory (DFT) study and designed three molecular models to illustrate pure carbon, carbon with nitrogen at the edge, which we called pyridinic nitrogen, and carbon with nitrogen substituting the central carbon atom, which we called quaternary nitrogen. Through theoretical calculation, we found nitrogen-doped carbon nanotubes exhibited stronger interaction with polysulfides and lithium sulfides. This is attributed to the adsorption of these polar sulfur species on the negatively charged nitrogen-doped sites. It revealed that nitrogen-doped carbon nanotube might worth trying."
"Then, we just prepared nitrogen-doped carbon nanotube/sulfur composites and assembled batteries to check if our theoretical results were reliable. Amazingly, the electrochemical experiment matched theoretical prediction very well. Comparing to raw carbon nanotube-based battery, the cycling life was significantly promoted by six times. Furthermore, delicate electrochemical analysis supported theoretical results and cell performance." said Hong-Jie. This work proposes the importance of a stable dynamic interface between carbon hosts and sulfur-containing guests and shed a new light on the lithium-sulfur battery decay mechanism, which was recently published in Advanced Material Interfaces.
"More advanced host materials satisfying the demand of amphiphilicity to both unpolar and polar sulfur species is going to be explored," Qiang said.
More information: Peng, H.-J., Hou, T.-Z., Zhang, Q., Huang, J.-Q., Cheng, X.-B., Guo, M.-Q., Yuan, Z., He, L.-Y. and Wei, F. (2014), "Strongly Coupled Interfaces between a Heterogeneous Carbon Host and a Sulfur-Containing Guest for Highly Stable Lithium-Sulfur Batteries: Mechanistic Insight into Capacity Degradation." Advanced Materials Interfaces. DOI: 10.1002/admi.201400227
Provided by Tsinghua University