(Phys.org) —Scientists from the Friedrich Miescher Institute for Biomedical Research have discovered how microRNAs repress translation of mRNAs. In a structure-function study published in Molecular Cell, they report different modes of recruitment of the CCR4-NOT complex to mRNAs targeted by microRNAs. They also demonstrate that CCR4-NOT recruits and activates the ATPase DDX6, an important translational inhibitor. Activation of DDX6 by CCR4-NOT plays a key role in miRNA repression.
As well as encoding information for protein synthesis, genomes of eukaryotic cells are transcribed into thousands of so-called non-coding RNAs, which perform a variety of essential structural and regulatory functions. Among the non-coding RNAs, one family is receiving particular attention – the ~20-nucleotide-long RNAs known as microRNAs (miRNAs). This family, comprising over 1,000 species, regulates the expression of more than half of all human genes. Moreover, dysfunction of miRNAs is associated with many human diseases, including cancer.
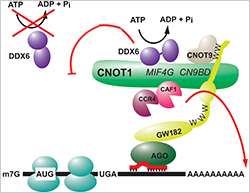
MiRNAs control gene expression post-transcriptionally, generally by imperfectly base-pairing to target messenger RNAs (mRNAs) and causing inhibition of their translation, as well as deadenylation and destabilization. They function as part of ribonucleoprotein particles (miRNPs), containing Argonaute (AGO) and GW182 family proteins as key components. While much progress has been made in understanding microRNA-induced mRNA degradation, it has remained unclear how miRNAs repress translation (i.e. protein synthesis), and several different mechanisms have been proposed.
Witold Filipowicz, emeritus Group Leader at the Friedrich Miescher Institute for Biomedical Research, and his group have already made significant contributions to the elucidation of possible modes of translational repression. They recently identified a large protein complex, CCR4-NOT, which is recruited to mRNA-associated miRNPs, thereby mediating not only mRNA deadenylation but also translational repression. They also showed that GW182 proteins interact directly with the CNOT1 scaffold subunit of CCR4-NOT, and that this interaction requires tryptophan (W) residues in W motifs dispersed across the repressive regions of GW182 proteins.
In a paper published recently in Molecular Cell, Hansruedi Mathys from the Filipowicz group and collaborators have now presented further details of the interaction of GW182 proteins with the CCR4-NOT complex. Filipowicz comments: "We found different modes of CCR4-NOT recruitment by GW182, which opens interesting avenues for intervention in miRNA-mediated repression."
In one mode, GW182-CNOT1 interaction is mediated by the CCR4-NOT subunit CNOT9. Determination of the crystal structure of the CNOT1-CNOT9 complex with bound tryptophans (mimics of GW182 W motifs) made it possible not only to reveal details of the interaction but also to design mutants used to test the relevance of the interaction. Filipowicz emphasizes: "For this and other aspects of the project, we were very fortunate to collaborate with Elena Conti's crystallography group at the Max Planck Institute of Biochemistry in Martinsried."
More importantly, Mathys and collaborators found that another sub-domain of CNOT1 with an MIF4G fold recruits the DEAD-box ATPase DDX6, which functions as a translational inhibitor. X-ray structures of DDX6 alone and its complex with the CNOT1 MIF4G, together with biochemical experiments, revealed that CNOT1 modulates the conformation of DDX6 and stimulates its ATPase activity. Filipowicz concludes: "We thus identified the long sought-after ligand of the DDX6 ATPase, and then showed that the CNOT1 MIF4G–DDX6 interaction and the ATPase activity of DDX6 are important for miRNA-mediated repression."
These findings provide initial structural and biochemical insights into the repressive steps downstream of the GW182 proteins and the role of the CCR4-NOT complex in post-transcriptional regulation in general.
More information: Mathys H, Basquin J, Ozgur S, Czarnocki-Cieciura M, Bonneau F, Aartse A, Dziembowski A, Nowotny M, Conti E, Filipowicz W (2014) "Structural and Biochemical Insights to the Role of the CCR4-NOT Complex and DDX6 ATPase in MicroRNA Repression." Molecular Cell (2014), DOI: 10.1016/j.molcel.2014.03.036
Journal information: Molecular Cell