An international team of researchers from the United States, France and Moscow Institute of Physics and Technology (Russia) has synthesized a previously unknown form of magnesium carbide. This material can be used for synthesizing carbon nanostructures and other compounds. Details can be found in an article published in the journal Inorganic Chemistry.
A team of researchers from the Carnegie Institution for Science (United States), Paris-Sorbonne University, the European Synchrotron Radiation Facility in Grenoble,the SOLEIL synchrotron facility (France), the State University of New York at Stony Brookin the United States, and MIPT has synthesized and studied samples of a substance named beta magnesium carbide (Mg2C3).
To synthesize the compound, the group used presses that are able to create pressures of up to several tens of GPa (hundreds of thousands of atmospheric pressures) and that can heat a sample to more than 1,000 degrees Celsius. Using X-ray analysis, NMR (nuclear magnetic resonance) and optical spectroscopy they collected data showing that this substance has a unique atomic structure.
Experiments showed that the new version of magnesium carbide retains its structure after pressure is reduced to normal and temperatures return to normal room temperatures. It is still too early to speak about any devices that could use the obtained substance, the researchers say, but they add that, nevertheless, Mg2C3 is a promising element for synthesizing other compounds, including various carbon nanostructures.
Chemistry and Synchrotrons
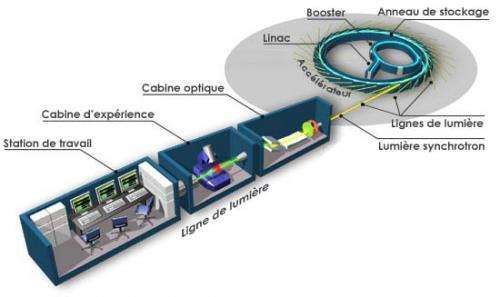
Originally intended for experiments in the field of elementary particle physics, accelerators have been an invaluable tool for research in many different areas. Turning a beam of charged particles produces X-rays, which exceeds the radiation from traditional cathode tubes (a standard X-ray source for medical equipment) by a number of parameters. Accelerators are capable of producing radiation many orders of magnitude brighter, providing record short impulses. Moreover, the radiation is monochromatic and with the required polarization.
Synchrotrons have enabled chemists to carry out X-ray diffraction analysis of samples of any nature, including both inorganic compounds and biomolecules. Modern accelerators can X-ray minerals, details of mechanisms and structures, archaeological artifacts, and determine the exact chemical composition of a sample. Furthermore, ultra short impulses of radiation allow scientists to take snapshots of certain phases of chemical reactions, "catching" short-living intermediate products.
Modern synchrotron radiation centers offer various pieces of equipment, including furnaces, presses and spectrometers.
Journal information: Inorganic Chemistry
Provided by Moscow Institute of Physics and Technology