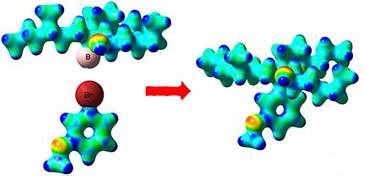
A boronic ester with a specific 3-D shape couples with an aromatic unit to create a new molecule which preserves the 3-D shape of the initial boronic ester in the final product
(Phys.org) —A new method for coupling together secondary and tertiary boronic esters to aromatic compounds which preserves the 3-D shape of the boronic ester is described by researchers from the University of Bristol in Nature Chemistry today. The method could have widespread application in the development of new, more effective drugs.
The method, discovered by Professor Varinder Aggarwal and colleagues in the School of Chemistry, fills a major gap in the Nobel prize-winning Suzuki-Miyaura reaction.
Over a third of all potential pharmaceuticals tested have employed this particular carbon-carbon (C-C) bond forming reaction. However, in the past, it has been limited to the creation of achiral, aromatic compounds, a class of essentially flat molecules which are now widely regarded as a major contributing factor to the failure of millions of biomolecules tested.
Thus, there is a major drive in the pharmaceutical industry to 'escape from flatland' as awareness grows of the importance of creating biomolecules with 3-D architectures which have much greater clinical success.
While many reliable methods are available for the easy synthesis of boronic esters with specific 3-D shape, there has been no way of using them to create useful C-C bonds to aromatic units.
The Bristol team have now found a unique method for achieving this that preserves the 3-D shape of the boronic ester in the final product. They believe that this reaction should find considerable application in academia and in industry.
More information: "Enantiospecific sp2–sp3 coupling of secondary and tertiary boronic esters." Amadeu Bonet, et al. Nature Chemistry (2014). DOI: 10.1038/nchem.1971
Journal information: Nature Chemistry
Provided by University of Bristol

.jpg)


















