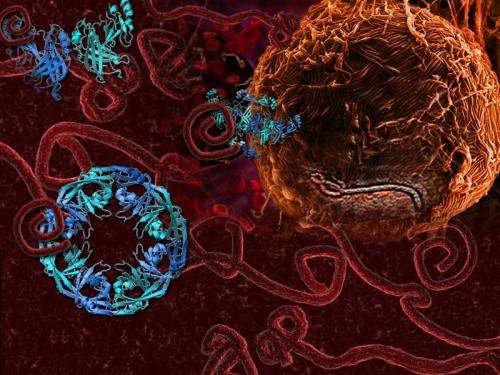
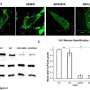
VP40, a protein of the Ebola virus, can arrange itself into three very different shapes, shown in blue, each with a distinct function. Credit: Nikola Stojanovic/SLAC and Zachary Bornholdt/The Scripps Research Institute
(Phys.org) —A new study reveals that a protein of the Ebola virus can transform into three distinct shapes, each with a separate function that is critical to the virus's survival. Each shape offers a potential target for developing drugs against Ebola virus disease, a hemorrhagic fever that kills up to 9 out of 10 infected patients in outbreaks such as the current one in West Africa.
At SLAC's Stanford Synchrotron Radiation Lightsource (SSRL) microbeam facility for crystallography, and other X-ray facilities, a team led by Erica Ollmann Saphire of The Scripps Research Institute analyzed the structure of VP40, a protein best known for its role in creating and releasing new copies of the virus from infected cells.
"The interesting thing about VP40 is that it does more than that," Saphire says. "We found that it is multifunctional, with several essential roles for the virus." The team reported its results in Cell.
One Protein, Three Structures
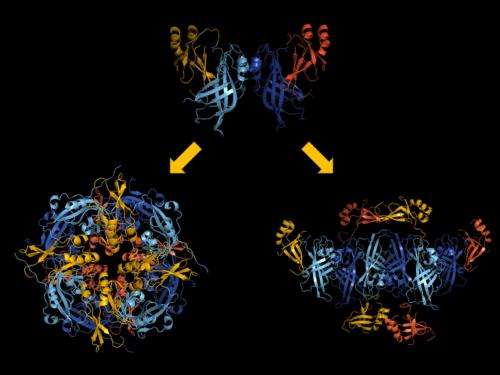
The team discovered that the protein can alter its shape, causing multiple copies of the protein to join up and create three very different assemblies: a butterfly shape composed of two, a ring formed by eight, and a linear structure built from six VP40 molecules. Prior to the study, only the protein ring was known.
But what unique functions do the individual structures have? The researchers took the study to the next level by combining their X-ray data with additional biological experiments. "This approach allowed us to track not only where the different structures are located, but also what they do inside the cell," Saphire says.
Each of VP40’s structural arrangements is linked to a different function in the virus life cycle. While traveling inside infected cells, VP40 assumes a butterfly shape (top). Near the cell nucleus, VP40 transforms into a ring (bottom left) that regulates how the viral genetic information is copied. At the cell membrane, VP40 assembles into a linear structure (bottom right), which plays a role in the creation of new viruses. Credit: Erica Ollmann Saphire and Zachary Bornholdt/The Scripps Research Institute
It turns out that the function of each structure is linked to a specific stage of the virus life cycle.
While moving around inside infected cells, VP40 assumes the butterfly shape.
In the early stages of an infection, the VP40 molecules change their structure and assemble into a ring near the cell nucleus, regulating how the virus's genetic information is copied.
In the later stages, VP40 travels to the cell's outer layer, or membrane, and transforms into its linear structure, which plays a crucial role in the creation of new copies of the virus.
The transformational changes of VP40 update the nearly 60-year-old "central dogma of biology," which implies that a given gene typically makes a single protein with a single 3-D shape. "Our findings open the central dogma wide up," says Saphire, who suggests that structural rearrangements as seen in VP40 may be more common than previously thought.
From an evolutionary perspective, structural diversity has developed out of necessity. Unlike humans, who possess some 20,000 protein-encoding genes, the Ebola virus must get by with a drastically smaller number.
Ebola Virus Protein: Butterfly to Ring.
"The Ebola virus has only seven genes. However, its proteins must serve many more functions than that," explains Scripps researcher Zachary Bornholdt, the study's first author. "Protein transformability allows the virus to make the most out of very little."
Potential Drug Targets
All three functions of VP40 – traveling inside infected cells, regulating genetic information and creating new viruses – are essential to the Ebola virus, and disrupting any of the corresponding structures or their transformations would severely affect it. Therefore, VP40's triple role provides researchers with important clues for the development of potential antiviral drugs.
"The more we are able to define VP40's structures and functions, the more we can expand what we can do with this information," Bornholdt says. "Our data suggest, for instance, that it might be more effective to target the ring than the other structures because only a small fraction of all VP40 molecules form the ring in the course of the viral life cycle."
Although a cure for Ebola virus disease is still remote, the new study already has practical applications: The same VP40 proteins produced for this study are being used in test strips to identify the disease in patients affected by the current outbreak in West Africa.
More information: Bornholdt et al., "Structural Rearrangement of Ebola Virus VP40 Begets Multiple Functions in the Virus Life Cycle." Cell 154, 763–774 (2013), DOI: 10.1016/j.cell.2013.07.015.
Journal information: Cell
Provided by Stanford University