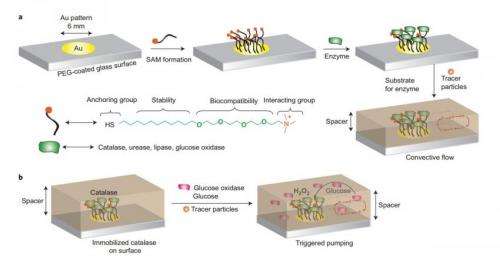
At catalase enzyme is immobilized on a gold platform. The enzyme pumps out fluid (which contains tracer particles to allow for observation) at a rate that is dependent on the concentration of glucose oxidase and glucose in the surrounding solution. Credit: Sengupta, et al. ©2014 Nature
(Phys.org) —For next-generation smart devices, autonomy is key. These devices will be able to power themselves, independently respond to stimuli, and perform different kinds of work, all without human intervention. With these abilities, smart devices could potentially have very wide-reaching implications.
In a recent study published in Nature Chemistry, Samudra Sengupta, et al., from The Pennsylvania State University, the Ural Branch of the Russian Academy of Sciences, and the University of Puerto Rico-Mayagüez, have designed and demonstrated a self-powered enzyme micropump that autonomously delivers small molecules and proteins in response to specific chemical stimuli.
"We demonstrate that surface-anchored enzymes can act as pumps in the presence of their respective substrates, pumping fluid and particles in a directional manner," coauthor Ayusman Sen, Professor of Chemistry at Penn State, told Phys.org. "This discovery enables the design of non-mechanical, self-powered nano/microscale pumps that precisely control flow rate and turn on in response to specific stimuli. One example described in the paper is the release of insulin from a reservoir at a rate proportional to ambient glucose concentration."
As a proof-of-principle, the researchers demonstrated how an enzyme micropump can be used to pump out insulin in response to the glucose concentration in the surrounding solution. A similar process occurs in the pancreas of healthy individuals, and afterwards the increased insulin stimulates muscle and fat cells to absorb the increased amounts of glucose from the blood.
However, in individuals with Type 1 diabetes, the pancreas does not produce sufficient amounts of insulin in response to elevated blood sugar levels. By autonomously releasing insulin in response to glucose concentration, the enzyme micropump essentially fulfills this role of the pancreas.
Demonstration of the fluid flow produced by a urease enzyme micropump in response to the presence of urea. Credit: Sengupta, et al. ©2014 Nature
The pump itself is relatively simple, consisting of a group of enzymes that are immobilized on a substrate. If the enzymes were not immobilized, the forces that they generate by releasing fluid would cause them to move around.
The researchers built enzyme micropumps using four kinds of enzymes (catalase, lipase, urease, and glucose oxidase), each of which responds to different chemical stimuli. For each case, the researchers observed that the pumping velocity is directly dependent on the stimulus concentration, enabling controllable delivery with no external power source.
"In living systems, the motors and pumps are powered by enzymes that convert ATP to ADP," Sen explained. "What we show is that one need not be tied to this one specific reaction, and that other enzymatic reactions can also generate a mechanical force for pumping. Our results open up a new area of mechanobiology: intrinsic force generation by non-ATP-dependent enzymes and their role in fluid transport in and outside biological systems."
For the case of the insulin-producing enzyme micropump, the researchers used a highly flexible hydrogel as a scaffold to serve two purposes: immobilize the enzymes, and trap and store the insulin molecules that will later be pumped out.
Although previous research has demonstrated passive insulin pumps that release insulin through scaffold decomposition, the active enzyme micropump has the advantages of releasing insulin at a rate proportional to the glucose concentration, as well as offering the possibility for being rechargeable. In addition, these self-powered pumps can remain viable and be capable of 'turning on' even after prolonged storage.
"Obviously, much more work and testing needs to be done to make the micropumps useful in diabetes therapy," Sen said. "I suppose that the hydrogel could be implanted in the body and they could be recharged by injecting more insulin into the gel reservoir. We have not yet completely worked all this out. The gel will also need to be tested for biocompatibility."
In addition to pumping insulin, enzyme micropumps could have many other applications. As one example, the pumps can be used to reduce the concentration of toxic substances. As the researchers explain, a pump can be triggered by a toxic substance, such as a nerve agent, that will then be drawn toward the pump and be consumed.
"The enzyme pump might use nerve agents as fuel and release an antidote in return," Sen said.
Currently, the researchers are working on expanding the enzyme micropump concept to involve multienzyme cascades, which could lead to microfluidic logic gates. Still other applications include inexpensive sensors (tracers or dyes in the fluid can be used to monitor fluid speed, which indicates the concentration of a biomarker or toxin), as well as particle assembly/disassembly.
"Since the enzyme pumps can pump particles suspended in a fluid, it should now be possible to form particle assemblies in specific locations by directional pumping," Sen said. "Furthermore, pumping can also be employed to disassemble such structures by directed transport of materials to specific places."
More information: Samudra Sengupta, et al. "Self-powered enzyme micropumps." Nature Chemistry. DOI: 10.1038/NCHEM.1895
Journal information: Nature , Nature Chemistry
© 2014 Phys.org