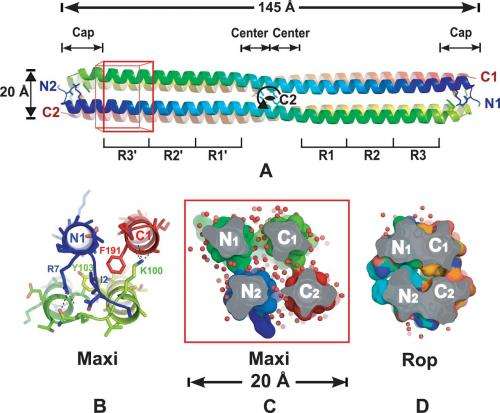
Four-helix bundle structure of Maxi has an open core. Credit: Science 14 February 2014: Vol. 343 no. 6172 pp. 795-798 DOI: 10.1126/science.1247407
(Phys.org) —A team of researches with Queen's University, in Canada has found that the antifreeze protein Maxi, defies the normal expectations of a protein by having a core filled with water molecules. In their paper published in the journal Science, describing their research with the protein, the team suggests that Maxi may cause scientists to rethink the mechanisms and kinetics of protein folding. Kim Sharp describes the research effort and the team's finding in a Perspective piece in the same journal.
Antifreeze proteins are useful because they absorb to the surface of ice crystals and in so doing, prevent them from growing—solutions that harbor them thus remain in a liquid state at temperatures below freezing. Proteins come about as a result of a process known as folding—aliphatic and aromatic side chains pack together forcing water out—without water in the core the protein prevents ice crystal formation. Because the cores that result are hydrophobic they are known as being anhydrous, and scientists believe they come about through one of two processes: evaporation or expulsion. In this new effort the researchers have turned the understanding of antifreeze protein folding on its head, as Maxi, when its formed via folding, doesn't force all the water out of its core, instead it retains 400 water molecules. This discovery has caused the research team to question whether the prevailing views regarding protein folding in general are correct.
With water in its core, it would seem that Maxi would allow ice crystal formation, but that's not the case, and it's because Maxi has hydrophobic molecules on the outside of its structure, thus the molecule appears almost as if inside out. With that the case, the question arises of how the protein arrives at its structure through folding? The researchers don't know but suspect it aligns more with the protein folding that normally follows the expulsion approach as it doesn't seem possible that such a protein could develop due to an evaporative process.
The researchers learned of the odd structure of Maxi by looking at it at 1.8-angstrom resolution—close enough to count the water molecules inside and to see that they were effectively trapped in a way that allowed for stabilization—the protein is longer than most antifreeze proteins. Sharp suggests that further study of Maxi will lead to a better understanding of the true mechanics of protein folding and ultimately a clearer view of protein formation in general.
More information:
An Antifreeze Protein Folds with an Interior Network of More Than 400 Semi-Clathrate Waters, Science 14 February 2014: Vol. 343 no. 6172 pp. 795-798
DOI: 10.1126/science.1247407
Abstract
When polypeptide chains fold into a protein, hydrophobic groups are compacted in the center with exclusion of water. We report the crystal structure of an alanine-rich antifreeze protein that retains ~400 waters in its core. The putative ice-binding residues of this dimeric, four-helix bundle protein point inwards and coordinate the interior waters into two intersecting polypentagonal networks. The bundle makes minimal protein contacts between helices, but is stabilized by anchoring to the semi-clathrate water monolayers through backbone carbonyl groups in the protein interior. The ordered waters extend outwards to the protein surface and likely are involved in ice binding. This protein fold supports both the anchored-clathrate water mechanism of antifreeze protein adsorption to ice and the water-expulsion mechanism of protein folding.
Journal information: Science
© 2014 Phys.org