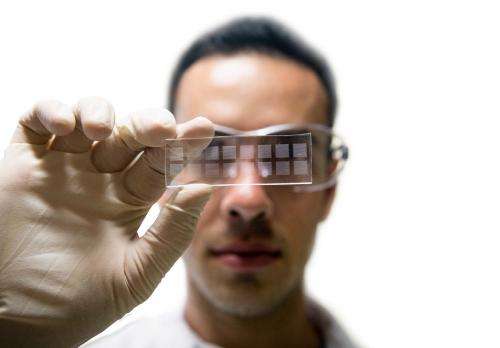
Jeff Gole (Bioengineering Ph.D. '13) holds arrays of 12-nanoliter-volume microwells. Confining genome amplification to individual microwells increases the effective template-genome concentration and improves amplification uniformity.
(Phys.org) —Bioengineers at the Jacobs School have created a better way to sequence genomes from individual cells. The breakthrough, which relies on microwells just 12 nanoliters in volume (see image), is one of many recent "omics" innovations from researchers across the Jacobs School and UC San Diego. The single-cell genome sequencing advance from Kun Zhang's lab could help researchers understand what causes Alzheimer's disease. The work could also enable scientists to identify tough-to-culture microbes living in ocean water and within the human body-by probing single cells.
The new sequencing approach, called MIDAS, yields genomes that exhibit comparatively little "amplification bias," which has been the most significant technological obstacle facing single-cell genome sequencing in the past decade. This bias refers to the fact that the amplification step is uneven, with different regions of a genome being copied different numbers of times. Minimizing bias is crucial for identifying meaningful differences between genomes, such as those found within different neurons from an individual with Alzheimer's or Parkinson's disease, or schizophrenia.
"Omics" Hotbed
Bioengineering and computer science researchers from UC San Diego and their collaborators are well established leaders in developing, implementing and teaching genomics and other "omics" technologies. Here are just a few recent examples. Shankar Subramaniam's bioengineering lab developed a new method for analyzing RNA transcripts-the transcriptome-from samples of 50 to 100 cells. The transcriptome serves as a proxy for which genes are being expressed and at what levels at a given moment. The new methods for assessing the transcriptome from small numbers of cells are already being applied to brain cancer, liver function and stem cell biology projects. This work is part of a shift-initiated in part here at UC San Diego-to approaching biological and health questions from a systems perspective.
"If you want to address a particular disease, the days of just looking into one gene, one protein or one signaling pathway are over. You need to look at all levels of complexity," said Vipul Bhargava (bioinformatics Ph.D. '13) the first author on the transcriptome paper.
Bernhard Palsson's research group has also been at the bleeding edge of systems biology for years. They recently used the genomic sequences of 55 E. coli strains to reconstruct the metabolic repertoire for each strain, work that could prove useful in developing ways to control deadly E. coli infections and to learn more about how certain strains of the bacteria become virulent. Meanwhile, processes developed by Genomatica, a sustainable chemicals company that spun out of the Palsson lab by alumnus Christophe Schilling, are being used by corporations around the world.
Provided by University of California - San Diego