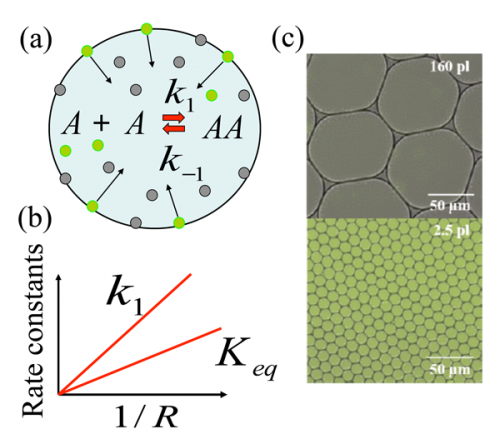
(a) Schematic of the reaction A+A⇔k1k-1AA in the aqueous droplet; grey circles represent nonfluorescent reactant A and green circles represent fluorescent product AA. Product AA generated at the surface desorbs into the droplet’s interior (as marked by arrows) resulting in enhanced synthesis. (b) The apparent forward reaction rate constant k1 and the apparent equilibrium constant Keq=k1/k-1 are inversely proportional to the droplet radius R. (c) Fluorescence imaging of droplets with radii 34 micrometers (upper image) and 8 micrometers (lower image) illustrates an increase in concentration of fluorescent product AA in the smaller droplets with the color change of these droplets with respect to the larger ones. Credit: Physics Viewpoint / Olga Kuksenok/University of Pittsburgh;(panel (c) adapted from Fallah-Araghi et al.)
(Phys.org) —A team made up of researchers from France, Germany, the U.K. and the U.S. has found that chemical reactions that result in the formation of new molecules tend to run much faster when confined to a very small space. In their paper published in Physical Review Letters, the team describes experiments they carried out that showed that certain chemical reactions that occurred in micro-droplets ran much faster than similar reactions occurring in a non-constricting environment.
The research by the team is part of an effort to try to learn how it was that life here on Earth came to be—likely as part of some unknown chemical reaction. To that end, they wondered if perhaps such reactions might have been more likely if they occurred in a more constricted space. Others have suggested that life may have gotten its start in micro-droplets of water after they evaporated from the ocean or other sources. To find out if such an idea might be plausible, the researchers tried to mimic conditions that existed on our prebiotic planet.
They chose two molecules—an aldehyde and an amine —taken alone they are not florescent—when they meet, though, a reaction occurs causing the formation of a molecule that is: a fluorescent imine. They conducted several experiments whereby the two molecules were allowed to meet and react in different sized liquid environments—from standing pools of water to micro-droplets. To measure how fast the reaction took place, the team measured how quickly the mix turned fluorescent. They report that the reactions occurring in the micro-droplets ran as fast as forty five times as fast as the reactions occurring in standing water.
To explain what they had observed, the team created a mathematical model that allows for manipulating volume size versus surface area of the environment. The model suggests that reactants in a small liquid volume where there is a relatively large surface area, provide an opportunity for the creation of larger molecules. That, the researchers suggest, might help explain how larger molecules (such as those that eventually led to something that was alive) could form from smaller ones during the time when life was beginning to emerge. Notable is that their experiments show that evaporation may not have been part of that process.
The team plans to next study multi-step reaction times in confined spaces.
More information: Enhanced Chemical Synthesis at Soft Interfaces: A Universal Reaction-Adsorption Mechanism in Microcompartments, Phys. Rev. Lett. 112, 028301 (2014) DOI: 10.1103/PhysRevLett.112.028301 (Free PDF)
Abstract
A bimolecular synthetic reaction (imine synthesis) was performed compartmentalized in micrometer-diameter emulsion droplets. The apparent equilibrium constant (Keq) and apparent forward rate constant (k1) were both inversely proportional to the droplet radius. The results are explained by a noncatalytic reaction-adsorption model in which reactants adsorb to the droplet interface with relatively low binding energies of a few kBT, react and diffuse back to the bulk. Reaction thermodynamics is therefore modified by compartmentalization at the mesoscale—without confinement on the molecular scale—leading to a universal mechanism for improving unfavorable reactions.
Journal information: Physical Review Letters
© 2014 Phys.org