The semiconductor industry of the future had high expectations of the new material silicene, which shares a lot of similarities with the 'wonder material' graphene. However, researchers of the MESA+ Research Institute of the University of Twente - who recently managed to directly and in real time film the formation of silicene - are harshly bursting the bubble: their research shows that silicene has suicidal tendencies. The research has been published by the renowned academic journal Applied Physics Letters.
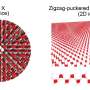
The material silicene was first created in 2010. Just like graphene, it consists of a single layer of atoms arranged in a honeycomb pattern. Graphene consists of carbon atoms, silicene of silicon atoms.
Because of their special properties - both materials are very strong, thin and flexible and have good electrical conductivity - graphene and silicene seem very well suited for the semiconductor industry of the future. After all, the parts on computer chips have to become smaller and smaller and the limits of the miniaturization of parts made of silicon are drawing closer and closer. The material silicene seems to be several steps ahead of graphene, because the semiconductor industry has been using silicon (which, like silicene, consists of silicon atoms) for many years now. In addition, it is easier to realize a so-called bandgap in silicene, which is a prerequisite for a transistor.
Suicide
Researchers of the MESA+ Research Institute of the University of Twente have, for the first time, managed to directly and in real time capture the formation of silicene on film. They let evaporated silicon atoms precipitate on a surface of silver, so that a nice, almost closed, singular layer of silicene was formed.
So far so good, but the moment that a certain amount of silicon atoms fall on top of the formed silicene layer, a silicon crystal (silicon in a diamond crystal structure instead of in a honeycomb structure) is formed, which triggers the further crystallization of the material; an irreversible process. From that moment, the newly formed silicon eats the silicene, so to speak.
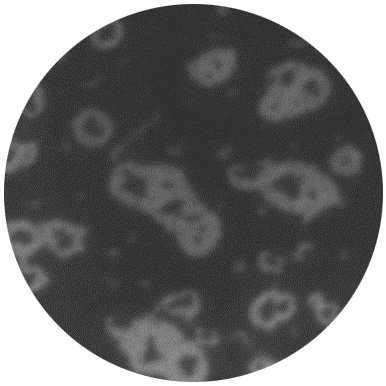
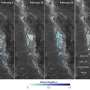
Video above: Formation of silicene on a silver surface (grey, start of the film). On top of the silver, silicene islands gradually start to form (black, halfway through the film). When the surface is almost completely covered, these collapse into silicon crystals again (black dots in grey areas, end of the film).
The reason for this is that the regular crystal structure (diamond) of silicon is energetically more favourable than the honeycomb structure of silicene and therefore more stable. Because of this property, the researchers did not succeed in covering more than 97 per cent of the silver surface with silicene, nor were they able to create multi-layered silicene. In other words: the moment a surface is almost completely covered with silicene, the material commits suicide and simple silicon is formed. The researchers do not expect it to be possible to create multi-layered silicene on a different type of surface, because the influence of the surface on the formation of the second layer of silicene is negligible.
More information: "The instability of silicene on Ag(111)." A. Acun, B. Poelsema, H. J. W. Zandvliet, R. van Gastel. Appl. Phys. Lett. 103, 263119 (2013); dx.doi.org/10.1063/1.4860964
Journal information: Applied Physics Letters
Provided by University of Twente