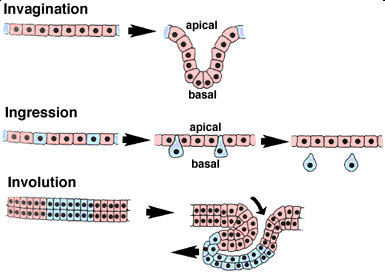
Key Events in Gastrulation. Credit: biology.kenyon.edu
(Phys.org) —Early embryonic development is a marvel of mechanics. Its signature step is the production of three tissue layers—mesoderm, ectoderm, and endoderm—through a topological maneuver known as gastrulation. While events that occur before, and after, this stage generally march to the beat of their own drum depending on the species of embryo, the invagination of external tissue to become internal tissue is a universally recognizable process. A recent paper published by Brunet et. al. in Nature Communications has now linked key conserved movements in gastrulation to specific molecular agents that make things happen at the critical hinge points.
Many genes have come and gone through the course evolution and the same holds true for those involved in early development. One thing that has become evident, is that without a properly functioning cytoskeleton, developing cells don't have the spine to pull off moves on the scale of the whole embryo. Brunet et. al. showed that when cytoskeletal function is perturbed early on, involution of the so-called ring cells, and subsequent gastrulation, is blocked. They also found that expression of the early mesodermal marker gene, ntl, was not activated in these ring cells.
With a move that would be worthy of Henry T. Brown's classic, 507 Mechanical Movements, the researchers injected these cells with tiny magnets and kick-started gastrulation by tractioning the ring cells with external magnets. Not only did normal development continue but expression of the normal mesodermal marker genes was detected. The researchers studied this phenomenon both in zebrafish and drosophila and were able to piece together a conserved pathway involving the cell adhesion protein B-catenin. They believe this dual function protein acts as a mechanosensor that responds to forces developed during gastrulation. It was found that when B-catenin is phosphorylated, it migrates from its membrane location into the nucleus where it acts to regulate gene transcription.
While direct quantification of the forces involved in gastrulation is difficult, new experimental techniques have recently been developed to infer these forces indirectly. By injecting special fluorocarbon oil droplets with fluorescent coatings, researchers can measure any deformation that occurs to the droplets as a result of any tensile or compressive forces in the surrounding tissue. The fluorocarbon used does not mix with cell membrane lipids permitting the droplets to remain intact.
It is likely that several mechanisms are involved here, although the authors feel that the phosphorylation of B-catenin could be a critical one. It may date back at least as far as the zebrafish - drosophila divergence epoch, or as long as 570 million years ago. Countless phosphorylation events continually occur throughout the cell and pinning such a critical event as gastrulation on any one of them would be a big claim. With techniques like micro-magnetic tractors and fluorescent fluorocarbon liposomes at your disposal, we might expect the hidden forces underlying development to soon give up their secrets.
More information: Evolutionary conservation of early mesoderm specification by mechanotransduction in Bilateria, Nature Communications 4, Article number: 2821 DOI: 10.1038/ncomms3821
Abstract
The modulation of developmental biochemical pathways by mechanical cues is an emerging feature of animal development, but its evolutionary origins have not been explored. Here we show that a common mechanosensitive pathway involving β-catenin specifies early mesodermal identity at gastrulation in zebrafish and Drosophila. Mechanical strains developed by zebrafish epiboly and Drosophila mesoderm invagination trigger the phosphorylation of β-catenin–tyrosine-667. This leads to the release of β-catenin into the cytoplasm and nucleus, where it triggers and maintains, respectively, the expression of zebrafish brachyury orthologue notail and of Drosophila Twist, both crucial transcription factors for early mesoderm identity. The role of the β-catenin mechanosensitive pathway in mesoderm identity has been conserved over the large evolutionary distance separating zebrafish and Drosophila. This suggests mesoderm mechanical induction dating back to at least the last bilaterian common ancestor more than 570 million years ago, the period during which mesoderm is thought to have emerged.
Perspective: www.nature.com/nature/journal/ … ll/504223a.html#ref4
Journal information: Nature Communications
© 2013 Phys.org