(Phys.org) —The discoloration of some pigments used in impressionist paintings has been observed since the 19th century. Belgian researchers have now investigated how the colors of chrome yellow pigments change on both the micro- and nanometer scales. In the journal Angewandte Chemie, they reveal the likely mechanism for the reaction that is slowly but surely changing the bright yellows of van Gogh's Sunflowers to an unsightly brown.
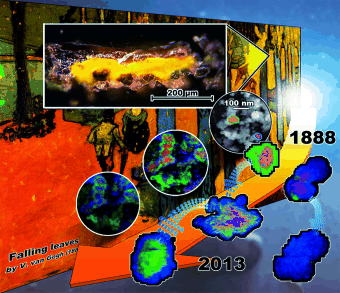
Many 19th century paintings, such as Les Alyscamps by Vincent van Gogh (1853–1890), contain synthetic chrome yellow pigments, primarily lead chromates. These yellow paints clearly turn brown when sunlight reduces chromium(IV) compounds present in the upper layers of paint to chromium(III). The degree to which the yellow paints discolor depends on the chemical composition and the crystal structure of the pigments. The most significant color changes are observed for chrome yellow pigments with a high sulfate content.
In order to develop useful techniques for the protection and restoration of paintings, it is important to understand the precise mechanisms that occur as the pigments darken. To solve this problem, Haiyan Tan, He Tian, and a team from the University of Antwerp examined samples from a 100-year-old tube of oil paint from the estate of the Flemish fauvist painter Rik Wouters (1882–1913). Their investigation revealed a variety of particles whose cores and shells differ in composition – clearly the early stages of an aging process. The researchers used scanning transmission electron microscopy and various spectroscopic methods to analyze the particles and to observe how they change when their aging is accelerated with UV light. The results allowed them to unlock the mechanisms behind the changes.
Originally, the paints consisted of a mixture of lead chromate, lead sulfate, and lead chromate sulfate particles in a linseed oil matrix. Lead sulfate particles are not affected by aging. The lead chromate sulfate particles are a different story: In the first step of the aging process, chromate ions dissolve in microscopic water droplets present between the particle and the binding agent. This happens more for particles with a high sulfate content because the chromium atoms in these particles are not as well-integrated into the crystal lattice. This process forms particles with a core made of lead chromate sulfate and a shell of lead sulfate. Irradiation with light favors the reaction of dissolved chromate with the linseed oil to form insoluble chromium oxide, Cr2O3, which is deposited in a third layer on the surface of the particle. More chromate ions dissolve out of the core, resulting in core–shell structures with a lead sulfate core and a chromium oxide shell.
As they age, the small lead chromate particles are completely reduced to chromium oxide, while larger ones maintain a core of lead chromate that is coated in a shell of chromium oxide. The dark chromium oxide thus slowly replaces and covers the yellow color of the lead chromate, causing the bright yellow areas in paintings to turn dark brown.
More information: Tian, H. Nanoscale Investigation of the Degradation Mechanism of a Historical Chrome Yellow Paint by Quantitative Electron Energy Loss spectroscopy Mapping of Chromium Species, Angewandte Chemie International Edition. dx.doi.org/10.1002/anie.201305753
Journal information: Angewandte Chemie , Angewandte Chemie International Edition
Provided by Angewandte Chemie