Tom22, the bouncer of the mitochondrion
Mitochondria burn sugar and supply the cell with energy. They were long thought to be structures that are relatively independent of the cell. However, Carolin Gerbeth, a PhD student from the trinational research training group "Membrane Proteins and Biological Membranes," has now identified no less than three signalling paths the cell uses to influence processes in the mitochondrion. In baker's yeast, she and her colleagues at the University of Freiburg found three enzymes that regulate the transport of proteins into the mitochondria. The team published its findings in the journal Cell Metabolism. "Our work lays an important foundation for investigating signalling pathways like these in humans and determining what role they play in the development of illnesses. In tumour cells the mitochondrial energy metabolism is dysregulated, and it is possible that this reprogramming is conveyed over these newly discovered signalling paths," explains project head Prof. Dr.. Chris Meisinger, Cluster of Excellence BIOSS Centre for Biological Signalling Studies and Institute of Biochemistry and Molecular Biology of the University of Freiburg.
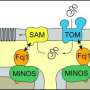
Mitochondria resemble a cell within the cell: Separated by two membranes from the rest of the cell and with their own genome, they were long thought to be regulated for the most part independently of the nucleus. However, most mitochondrial proteins are read off from the DNA in the nucleus and need to be transported to the mitochondria following their synthesis in the cytosol, the liquid surrounding the cell components. The mitochondrial proteins need to be sorted precisely according to their destination. Not just anything is allowed to pass through the membrane of the cellular powerhouses: Only with a molecular mailing address can a protein pass though the central entrance gate in the outer mitochondrial membrane, the TOM complex. In addition to the molecular pore Tom40, the complex contains receptors like Tom22, which decides in the manner of a bouncer at a nightclub which proteins can enter and which can't. The right "outfit" for admission is a particular molecular structure.
Not only do proteins found in the mitochondrion enter from outside: The entrance gate is also regulated by cellular proteins, as Gerbeth discovered. She demonstrated that two of these so-called kinases can make the gate more or less permeable by connecting a phosphate to the precursor of the protein Tom22. The catalyst in this case is the sugar glucose, which uses the yeast as food and as an source of energy. This enables the yeast cell to adjust the energy metabolism in the mitochondrion to changes in the environment. When glucose is available in abundance, for example, the pores allow fewer proteins, which are necessary for energy production, to pass through - the mitochondria shift into economy mode and the cell obtains its energy from the cytosol. One of the kinases is even embedded directly in the outer membrane, right next to the TOM complex. Up to now, only few protein kinases have been found in mitochondria.
More information: Gerbeth, C. et al. Glucose-Induced Regulation of Protein Import Receptor Tom22 by Cytosolic and Mitochondria-Bound Kinases (2013), Cell Metabolism, Volume 18, Issue 4, 578-587. DOI: 10.1016/j.cmet.2013.09.006
Journal information: Cell Metabolism
Provided by Albert Ludwigs University of Freiburg