Many of today's technologies, from hybrid car batteries to flat-screen televisions, rely on materials known as rare earth elements (REEs) that are in short supply, but scientists are reporting development of a new method to recycle them from wastewater. The process, which is described in a study in the journal ACS Applied Materials & Interfaces, could help alleviate economic and environmental pressures facing the REE industry.
Zhang Lin and colleagues point out that REEs, such as terbium—a silvery metal so soft it can be cut with a knife—behave in unique ways as super magnets, catalysts or superconductors. That makes them irreplaceable in many of today's tech gadgets and machines. Market watchers expect global demand to rise to at least 185,000 tons by 2015. Although some of these elements are actually plentiful, others are indeed in short supply. According to reports, terbium and dysprosium supplies may only last another 30 years. Attempts so far to recycle them from industrial wastewater are expensive or otherwise impractical. A major challenge is that the elements are typically very diluted in these waters. The team knew that a nanomaterial known as nano-magnesium hydroxide, or nano-Mg(OH)2, was effective at removing some metals and dyes from wastewater. So they set out to understand how the compound worked and whether it would efficiently remove diluted REEs, as well.
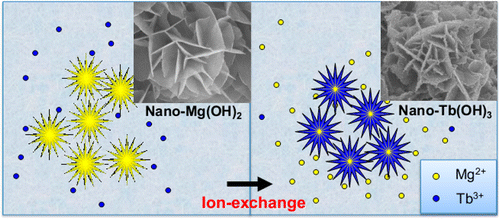
To test their idea, they produced inexpensive nano-Mg(OH)2 particles, whose shapes resemble flowers when viewed with a high-power microscope. They showed that the material captured more than 85 percent of the REEs that were diluted in wastewater in an initial experiment mimicking real-world conditions. "Recycling REEs from wastewater not only saves rare earth resources and protects the environment, but also brings considerable economic benefits," the researchers state. "The pilot-scale experiment indicated that the self-supported flower-like nano-Mg(OH)2 had great potential to recycle REEs from industrial wastewater."
More information: "Recycling Rare Earth Elements from Industrial Wastewater with Flowerlike Nano-Mg(OH)2" ACS Appl. Mater. Interfaces, 2013, 5 (19), pp 9719–9725. DOI: 10.1021/am4027967
Abstract
Treatment of wastewater containing low-concentration yet highly-expensive rare earth elements (REEs) is one of the vital issues in the REEs separation and refining industry. In this work, the interaction and related mechanism between self-supported flowerlike nano-Mg(OH)2 and low-concentration REEs wastewater were investigated. More than 99% REEs were successfully taken up by nano-Mg(OH)2. Further analysis revealed that the REEs could be collected on the surface of Mg(OH)2 as metal hydroxide nanoparticles (<5 nm). An ion-exchange model was proposed as a critical factor for both guaranteeing the reaction speed and maintaining the self-supported structure of the materials. In addition, a method was developed to further separate the immobilized REEs and the residual magnesium hydroxide by varying the solution pH. In a pilot-scale experiment, the REEs from practical wastewater were immobilized effectively at a high flow rate. We anticipate this work can provide a good example for the recycling of valuable REEs in practical industrial applications.
Journal information: ACS Applied Materials and Interfaces
Provided by American Chemical Society