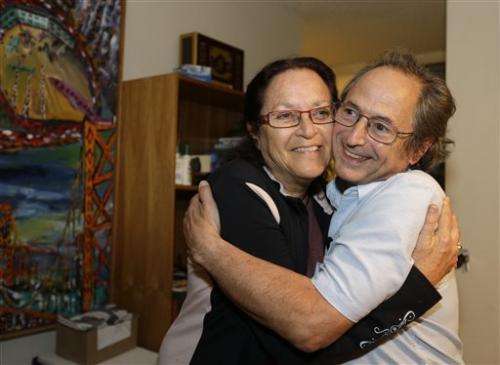This Wednesday Oct. 9, 2013 photo shows a webpage showing the laureates Martin Karplus, Michael Levitt and Arieh Warshel as winners of the 2013 Nobel Prize in chemistry, announced by the Royal Swedish Academy of Sciences in Stockholm. The prize was awarded for laying the foundation for the computer models used to understand and predict chemical processes. (AP Photo/Claudio Bresciani)
Three U.S.-based scientists won this year's Nobel Prize in chemistry on Wednesday for developing powerful computer models that researchers use to understand complex chemical interactions and create new drugs.
Research in the 1970s by Martin Karplus, Michael Levitt and Arieh Warshel has led to programs that unveil chemical processes such as how exhaust fumes are purified or how photosynthesis takes place in green leaves, the Royal Swedish Academy of Sciences said. That kind of knowledge makes it possible to find the best design for things like new drugs, solar cells or catalytic converters for cars.
The strength of the winning work is that it can be used to study all kinds of chemistry, the academy said.
"This year's prize is about taking the chemical experiment to cyberspace," said Staffan Normark, the academy's secretary.
All three scientists became U.S. citizens. Karplus, an 83-year-old U.S. and Austrian citizen, splits his time between the University of Strasbourg, France, and Harvard University. The academy said Levitt, 66, is a British, U.S., and Israeli citizen and a professor at Stanford University School of Medicine. Warshel, 72, is a U.S. and Israeli citizen affiliated with the University of Southern California in Los Angeles.
Levitt told The Associated Press the award recognized him for work he had done when he was 20, before he even had his PhD.
"It was just me being in the right place at the right time and maybe having a few good ideas," he said, speaking by telephone from his home in Stanford, California.
"It's sort of nice in more general terms to see that computational science, computational biology is being recognized," he added. "It's become a very large field and it's always in some ways been the poor sister, or the ugly sister, to experimental biology."
Jokingly, he said the biggest immediate impact of the Nobel Prize would be his need for dance lessons before appearing at the Nobel banquet.
"I would say the only real change in my life is I need to learn how to dance because when you go to Stockholm you have to do ballroom dancing," Levitt said. "This is the big problem I have right now."
Stanford University professor Michael Levitt, who won the Nobel Prize in chemistry, speaks during an interview at his home Wednesday, Oct. 9, 2013, in Stanford, Calif. Three U.S.-based scientists won this year's Nobel Prize in chemistry for developing powerful computer models that researchers use to understand complex chemical interactions and create new drugs. Research in the 1970s by Levitt, Martin Karplus and Arieh Warshel led to programs that unveil chemical processes such as how exhaust fumes are purified or how photosynthesis takes place in green leaves. (AP Photo/Eric Risberg)
Karplus told the AP the 5 a.m. call from the Nobel judges had him worried that the caller might be bearing bad news.
"Usually you think when you get a call at 5 o'clock in the morning it's going to be bad news, you know, something's happened. My daughter, you know, who is in Israel, might have been run over by a car or something or other.
"But it turned out to be good news and, after a while, I finally understood it was a call from Sweden and . I had been awarded a Nobel Prize in chemistry with two other people," Karplus said.
Warshel, speaking by telephone to a news conference in Stockholm, said he was "extremely happy" to have been woken up in the middle of the night in Los Angeles to find out he would share the $1.2 million prize, and looks forward to collecting it in the Swedish capital.
"In short, what we developed is a way which requires computers to look, to take the structure of the protein and then to eventually understand how exactly it does what it does," Warshel said.
When scientists wanted to simulate complex chemical processes on computers, they used to have to choose between software that was based on quantum physics, which applies on the scale of an atom, or classical Newtonian physics, which operates at larger scales. The academy said the three laureates developed computer models that "opened a gate between these two worlds."
Stanford University professor Michael Levitt, who won the Nobel Prize in chemistry, is embraced by his wife Rina at their home on Wednesday, Oct. 9, 2013, in Stanford, Calif. Three U.S.-based scientists won this year's Nobel Prize in chemistry for developing powerful computer models that researchers use to understand complex chemical interactions and create new drugs. Research in the 1970s by Levitt, Martin Karplus and Arieh Warshel led to programs that unveil chemical processes such as how exhaust fumes are purified or how photosynthesis takes place in green leaves. (AP Photo/Eric Risberg)
While quantum mechanics is more accurate, it's impossible to use on large molecules because the equations are too complex to solve. By using quantum mechanics only for key parts of molecules and classical physics for the rest, the blended approach delivers the accuracy of the quantum approach with manageable computations.
Working together at Harvard in the early 1970s, Karplus and Warshel developed a computer program that brought together classical and quantum physics. Warshel later joined forces with Levitt at the Weizmann institute in Rehovot, Israel, and at the University of Cambridge in Britain, to develop a program that could be used to study enzymes.
Jeremy Berg, a professor of computational and systems biology at the University of Pittsburgh, said the winning work gives scientists a way to understand complicated reactions that involve thousands to millions of atoms.
"There are thousands of laboratories around the world using these methods, both for basic biochemistry and for things like drug design," said Berg, former director of the National Institute of General Medical Sciences in Bethesda.
Martin Karplus poses at his home in Cambridge, Mass., after being awarded the Nobel Prize in chemistry Wednesday, Oct. 9, 2013. Karplus, who splits his time between Harvard and the University of Strasbourg, France, is among three awarded the Nobel Prize in chemistry for developing powerful computer models that any researcher can use to understand complex chemical interactions and create new drugs. (AP Photo/Josh Reynolds)
Many drug companies use computer simulations to screen substances for their potential as medicines, which lets them focus their chemistry lab work on those that look promising, he said.
Marinda Li Wu, president of the American Chemical Society, was equally enthusiastic about the award.
"I think it's fabulous," she said in a telephone interview. "They're talking about the partnering of theoreticians with experimentalists, and how this has led to greater understanding."
That is "bringing better understanding to problems that couldn't be solved experimentally," she said. "We're starting as scientists to better understand things like how pharmaceutical drugs interact with proteins in our body to treat diseases. This is very, very exciting."
Earlier this week, three Americans won the Nobel Prize in medicine for discoveries about how key substances are moved around within cells and the physics award went to British and Belgian scientists whose theories help explain how matter formed in the universe after the Big Bang.
The Nobel Prize in literature will be announced on Thursday, the Nobel Peace Prize on Friday and the economics prize on Monday.
All Nobel Prizes will be presented to the winners on Dec. 10, the anniversary of prize founder Alfred Nobel's death in 1896.
The Noble Committee Prize Announcement
The Royal Swedish Academy of Sciences has decided to award the Nobel Prize in Chemistry for 2013 to Martin Karplus (Université de Strasbourg, France and Harvard University, Cambridge, MA, USA), Michael Levitt (Stanford University School of Medicine, Stanford, CA, USA), and Arieh Warshel (University of Southern California, Los Angeles, CA, USA) "for the development of multiscale models for complex chemical systems"
The computer—your Virgil in the world of atoms
Chemists used to create models of molecules using plastic balls and sticks. Today, the modelling is carried out in computers. In the 1970s, Martin Karplus, Michael Levitt and Arieh Warshel laid the foundation for the powerful programs that are used to understand and predict chemical processes. Computer models mirroring real life have become crucial for most advances made in chemistry today.
Chemical reactions occur at lightning speed. In a fraction of a millisecond, electrons jump from one atomic nucleus to the other. Classical chemistry has a hard time keeping up; it is virtually impossible to experimentally map every little step in a chemical process. Aided by the methods now awarded with the Nobel Prize in Chemistry, scientists let computers unveil chemical processes, such as a catalyst's purification of exhaust fumes or the photosynthesis in green leaves.
The work of Karplus, Levitt and Warshel is ground-breaking in that they managed to make Newton's classical physics work side-by-side with the fundamentally different quantum physics. Previously, chemists had to choose to use either or. The strength of classical physics was that calculations were simple and could be used to model really large molecules. Its weakness, it offered no way to simulate chemical reactions. For that purpose, chemists instead had to use quantum physics. But such calculations required enormous computing power and could therefore only be carried out for small molecules.
This year's Nobel Laureates in chemistry took the best from both worlds and devised methods that use both classical and quantum physics. For instance, in simulations of how a drug couples to its target protein in the body, the computer performs quantum theoretical calculations on those atoms in the target protein that interact with the drug. The rest of the large protein is simulated using less demanding classical physics.
Today the computer is just as important a tool for chemists as the test tube. Simulations are so realistic that they predict the outcome of traditional experiments.
A look at the winners of Nobel Prize in chemistry
WHO WON?
Austrian-American Martin Karplus, 83, a professor of chemistry at the Universite de Strasbourg, France, and Harvard University; Michael Levitt, 66, a professor at Stanford University School of Medicine, who has American, Israeli and British citizenship; and Israeli-American Arieh Warshel, 72, a distinguished professor of chemistry at the University of Southern California.
FOR WHAT?
For creating computer models that help scientists better understand and predict complex chemical processes. They developed a program that blended classical physics, which works on a larger scale, with quantum physics, which works on the scale of an atom, leading to much more accurate results.
SIGNIFICANCE
It allowed chemists to simulate how molecules act in all kinds of environments, vastly speeding up the development of everything from new drugs to solar panels to catalytic converters in cars.
WHAT THEY SAID
Warshel: "You could use it, for example, to design drugs, or just, like in my case, to satisfy your curiosity."
Levitt: "It was just me being in the right place at the right time and maybe having a few good ideas."
More information: www.nobelprize.org/nobel_prize … s/2013/advanced.html
© 2013 The Associated Press. All rights reserved.