Strong forces at work in simple table salt
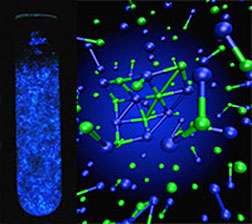
(Phys.org) —Inside the chemical processes to synthesize simple table salt crystals, or NaCl, intense electric fields, typically associated with particle accelerators, occur, according to Pacific Northwest National Laboratory scientists. The 5 GV/m fields can alter the NaCl solution's electronic structure. These findings are the next step in determining the exact mechanism underlying salt's crystallization and the long-lived cobalt blue light emitted (luminescence) during salt formation.
"The fields in the reactions are intense and can have dramatic effects on the electronic states inside the system," said Dr. Shawn Kathmann, the chemical physicist who led the study.
The ultimate goal is to control the synthesis of matter to make materials capable of innovative leaps in capturing, storing, and transducing energy. Exquisite control over material synthesis could lead to solar cells that capture more light to improve their efficiency or catalytic nanoparticles with significantly less precious metals, reducing financial and environmental costs. This level of control requires a fundamental understanding of synthesis at the molecular and electronic levels.
"Our goal is to control the synthesis of matter to make whatever we want, whenever we want, out of whatever we want," said Kathmann.
When NaCl crystallizes out of water at room temperature with no additional energy added, it emits a cobalt blue glow, indicating that electronic transitions are occurring and in direct contradiction to the general belief that salt crystallization does not involve a change in electronic structure or that the NaCl retains its ionic character.
"The basic point is that if luminescence occurs, something very different is actually happening than what we think is happening," said Dr. Bernhard Sellner, a PNNL postdoctoral fellow and a theoretical chemist on the study. "A photon of light cannot be emitted unless something exciting is happening to the electrons."
Using large-scale molecular dynamics simulations on NaCl solutions at the National Energy Research Scientific Computing Center, the team determined the fluctuations in the charges, electric potentials and fields.
"This is the first time these fields have been quantified and analyzed," said Dr. Marat Valiev, a PNNL chemical physicist and theorist on the project who works in EMSL.
In addition, the team analyzed the charge redistribution of the salt in aqueous solution as a function of concentration. They wanted to know if the charges on the water molecules and ions were what they expected. They discovered that the ion charges are not equal and opposite to each other. Some of the electronic charge on the chloride ion (Cl-) ends up on the water molecules in the first solvation shells around the chloride and sodium ions, with the waters around sodium being the most negative—the waters effectively act as an electronic sink.
The next step is to understand how long these intense fields last in aqueous electrolytes, determine what types of molecular configurations are needed to create these long-lasting fields, and quantify the distribution of energy gaps between the various excited electronic states. The near-term goal is to understand the electronic mechanisms of the process and the influence of trace impurities, such as silver or copper.
More information: Sellner B, M Valiev, and SM Kathmann. 2013. "Charge and Electric Field Fluctuations in Aqueous NaCl Electrolytes." Journal of Physical Chemistry B. In advance of publication. Cover article.
Journal information: Journal of Physical Chemistry B
Provided by US Department of Energy