September 18, 2013 report
Dissecting the brain's primary developmental engine
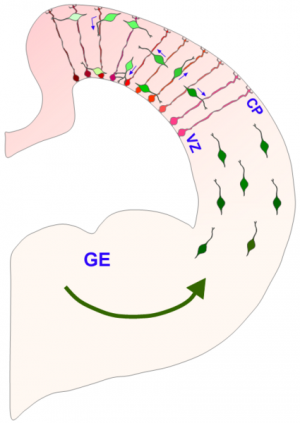
(Phys.org) —Last month, researchers reported the creation of the first primitive brain-like structures made from human stem cells. To create the complex morphology of these cerebral organoids, cells within a proliferating neuroectodermal layer were converted into so-called radial glial precursors (RGPs), which then rough out the basic floor plan of the cerebrum. As seen in the image above, the migrating and replicating RPGs span the thickness of the telencephalon in a normally developing brain, and provide a scaffolding for later arriving cells to subsequently migrate on themselves. A new paper published in Cell describes how the division of RPGs is tightly controlled by the location of the nucleus as it is motored about between the poles of the extended cell. The authors expand upon earlier work detailing the precise cytoskeletal couplers which power these translocations in specific directions, and ultimately piece together how these motor proteins, specifically dynein, are harnessed by nuclear pore complexes. Once this motor-nuclear mating occurs, migration in the apical direction (in this context, apical is towards the ventricle and basal is towards the surface) is initiated followed by subsequent entry into mitosis.
While the nucleus undergoes dramatic restructuring throughout the cell cycle, the individual pore complexes themselves are among the longest-lived protein structures in the cell. Together with a few other kinds of proteins, like the collagens, or specific histones, they can maintain their structure over the lifetime of the organism itself. In their latest research, the researchers were able to identify a number of proteins that inhibited the recruitment of dynein to the pore complex using a technique known as RNA interference (RNAi). The particular form of the technique they employed here made use of more stable RNA known as shRNA, for short hairpin. By injecting these inhibitors directly in utero, at embryonic day 16 rats, they then looked at the effects on RGP cells in living slices of tissue from embryonic day 20.
The researchers found that the interfering RNAs arrested the cells in the G2 phase of the cycle. By rebuilding and adding supplementary dynein proteins, and targeting them to the nuclear pore, this effect could be rescued. The specially modified dynein dramatically increased the number of RGP cells found at the ventricular surface. The authors suggest that the amount of dynein recruited to the nuclear envelope region was therefore sufficient to overcome the opposing forces generated kinesin-3 which are responsible for basal nuclear migration during the G1 phase.
The authors observed that the centrosomes remained at the ventricular surface during the periods of nuclear foraging to the basal pial layer (pia is the membranous covering of the brain). Their findings that the motor complex is instead associated with the nucleus in the RGP cells represents an uncommon mechanism. Why this mechanism of tightly coupled migration and mitotic entry evolved is currently unknown but the authors speculate that ectopic cell divisions must somehow interfere with proper neurogenesis.
Nuclear migration to the ventricular surface provides direct access to the centrosomes which are assembling the mitotic spindle, and also to additional cues that appear to specify cell fate. The exact orientation of this spindle apparatus has emerged as an important regulator of cell fate, and the subsequent migration of neural and glial progeny. Further exploration of this system may give additional clues about the mechanisms that ultimately specify the size and particular geometry of the cortex.
More information: Dynein Recruitment to Nuclear Pores Activates Apical Nuclear Migration and Mitotic Entry in Brain Progenitor Cells, Cell, Volume 154, Issue 6, 1300-1313, 12 September 2013. DOI: 10.1016/j.cell.2013.08.024
Journal information: Cell
© 2013 Phys.org