Efforts to develop a safer form of acetaminophen—the pain and fever-reducer that is one of the most widely used drugs—have led to discovery of substances that may have less potentially toxic effects on the liver. A report on the research appears in ACS Medicinal Chemistry Letters.
Roman Shchepin and colleagues explain that a link exists between acetaminophen and liver damage. The damage may be severe and can occur with intentional and accidental overdoses, as well as when susceptible individuals take the drug. Indeed, acetaminophen has been implicated in almost 50 percent of all acute liver failure cases in the United States alone. Scientists have known the biochemical basis of acetaminophen's liver toxicity, and Shchepin and colleagues set out to develop safer versions of acetaminophen.
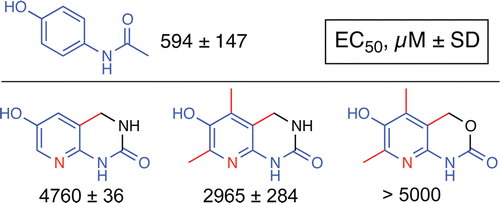
They describe the design and testing of two compounds that have a similar architecture to acetaminophen, but aren't toxic to liver cells grown in the laboratory. The researchers say that, although further testing is needed, these compounds are promising candidates for acetaminophen replacements.
More information: "Rational Design of Novel Pyridinol-Fused Ring Acetaminophen Analogues" ACS Med. Chem. Lett., Article ASAP DOI: 10.1021/ml4000904
Abstract
Acetaminophen (ApAP) is an electron donor capable of reducing radicals generated by redox cycling of hemeproteins. It acts on the prostaglandin H synthases (cyclooxygenases; COXs) to reduce the protoporphyrin radical cation in the peroxidase site of the enzyme, thus preventing the intramolecular electron transfer that generates the Tyr385 radical required for abstraction of a hydrogen from arachidonic acid to initiate prostaglandin synthesis. Unrelated to this pharmacological action, metabolism of ApAP by CYPs yields an iminoquinone electrophile that is responsible for the hepatotoxicity, which results from high doses of the drug. We synthesized novel heterocyclic phenols predicted to be electron donors. Two of these inhibited the oxygenation of arachidonic acid by PGHS-1 and myoglobin and also were shown to be more metabolically stable and exhibited less direct cytotoxicity than acetaminophen. They are leading candidates for studies to determine whether they are free of the metabolism-based hepatotoxicity produced by acetaminophen.
Journal information: ACS Medicinal Chemistry Letters
Provided by American Chemical Society