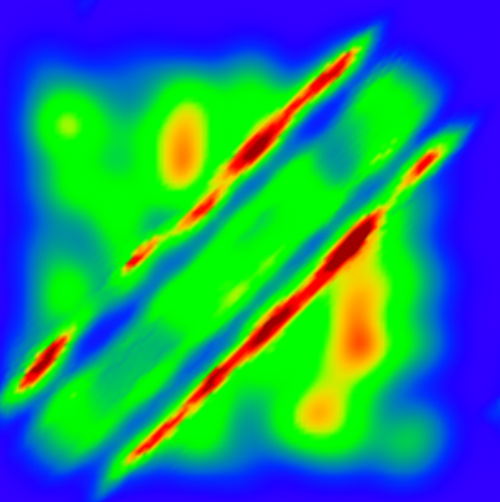
An image showing the probability distribution of two atoms forming the novel bond. Red color indicating high probability occurs at lines of fixed distance between the atoms. Credit: M. Lemeshko, H. Weimer
Basic chemistry tells us that a bond between atoms can form if it is energetically more favorable for the atoms to stick together than staying apart. This fundamentally requires an attractive force between the atoms. However, new theoretical predictions show that the combination of a repelling force and controlled noise from an environment can also have the surprising effect of leading to a bound state, although one with quite exotic properties. The research team consisting of Harvard postdoc Mikhail Lemeshko and former Harvard postdoc Hendrik Weimer report their results in the journal Nature Communications.
How is it possible that repulsion and noise, both two effects countering the formation of a chemical bond, can lead to a bound state nevertheless? To understand this, one has to take into account the quantum properties of the atoms: adding controlled noise to a quantum system can result in an interference phenomenon that traps the atoms in a well-defined quantum state. The repulsive force then ensures that this trapping occurs at a particular distance, which sets the length of the bond. The nature of the novel bound state is strikingly different from their chemical counterparts. For example, the bound state is remarkably robust and can hardly be broken by depositing a constant amount of energy to it.
Lemeshko and Weimer consider one of the most basic and universally available sources of noise: vacuum fluctuations of the electromagnetic field. In the past, the techniques making use of these quantum fluctuations have led to dramatic improvements in laser cooling, culminating in the results that were awarded the 1997 Nobel Prize in Physics. The authors believe that the first applications of the discovered binding mechanism might be in the area of cooling of atomic quantum gases.
More information: M. Lemeshko, H. Weimer. Dissipative binding of atoms by non-conservative forces. Nature Communications 4, 2230 (2013), available online at dx.doi.org/10.1038/ncomms3230
Journal information: Nature Communications
Provided by Harvard University