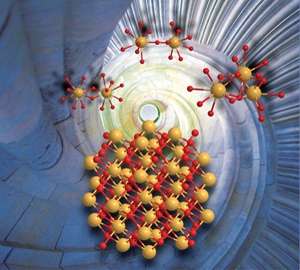
Cerium (IV) dimers and trimers form in aqueous solution nanometer-sized cerium dioxide crystals (CeO2). The size of the nanocrystals is in the order of two to three nanometers. Credit: Dr Atsushi Ikeda-Ohno
A simplified technique to fabricate nano-crystals of cerium dioxide (CeO2), which have wide-ranging technological and industrial applications, has been "unexpectedly" demonstrated by a UNSW chemist.
The UNSW-led study reveals that nano-crystals form naturally when a precursor material – Cerium (IV) – is dissolved and hydrolysed in water. It is the first time this formation process has been observed.
The findings, reported in Chemistry – A European Journal, could simplify the existing production process, which requires heating and the addition of chemicals to better control the crystals' shape and size.
"The most important finding is that cerium (IV) has an intrinsic nature to form uniformly sized nano-crystals of cerium dioxide of approximately two to three nanometres in an aqueous solution via hydrolysis," says lead author Dr Atsushi Ikeda-Ohno from the UNSW School of Civil and Environmental Engineering.
"The outcomes of this study provide a basic concept to simplify and alleviate the production process and means we only need to adjust the pH of the aqueous solution… without heating or adding chemicals," he says. "This could save money on energy costs and help reduce the environmental impact of the whole production process."
Cerium dioxide nano-crystals are formed from the rare earth element Cerium. They are used as catalysts to treat hazardous gases – converting toxic fumes into less harmful emissions; as electrodes in fuel cells; and in sunscreens and cosmetics due to their capacity to absorb high levels of UV radiation.
There is growing interest in the fabrication of these materials, given their wide-ranging applications, but there is relatively little known about the mechanisms that govern their formation. These mechanisms are directly linked to the ability to control their shape and size – features that govern a crystal's functionality.
One of the largest barriers to studying these formation mechanisms has been a lack of analytical tools to do so in-situ, says Ikeda-Ohno.
His study was initially focused on observing the effect of hydrolysis on Cerium (IV) and developing an improved analytical strategy, using a combination of spectroscopic tools, to observe and better understand the formation process. But then something unexpected happened.
"When I first investigated apparently-transparent solutions of Cerium (IV) at different pH by using an X-ray technique I realised that the solutions were not composed of simple dissolved species…but contained very tiny colloidal particles which are not visually recognisable," he said.
After applying additional spectroscopic and microscopic techniques to characterise these mysterious particles, he determined that they were in fact cerium dioxide nano-crystals.
"As a consequence, we have succeeded in observing the whole evolution process from the precursor species into Cerium dioxide nano-crystals," the report states.
"In this study I have demonstrated that a combination of advanced analytical techniques, particularly synchrotron-based X-ray techniques… provide a very powerful tool to probe the evolution of metal nano-crystals in-situ," says Ikeda-Ohno.
"A full understanding of the 'evolution' process from the precursor species into the resultant nano-crystals possibly enables us to tailor these materials to practical needs."
The next step toward realising the hydrolysis-based fabrication technique is to identify the critical pH condition at which the nano-crystals begin to form. Ikeda-Ohno says the approach he's developed could be used to that end, and could also be applied to other metal nano-crystals.
More information: onlinelibrary.wiley.com/doi/10 … m.201204101/abstract
Journal information: Chemistry – A European Journal
Provided by University of New South Wales