July 5, 2013 report
German scientists solve nonclassical 2-norbornyl carbocation structure
(Phys.org) —A team of chemists working in Germany has finally, after decades of debate, solved the crystal structure of the nonclassical 2-norbornyl carbocation. In their paper published in the journal Science, the team describes the arduous process involved in the work they did that led to the eventual determination of the nonclassical crystal structure of the ion.
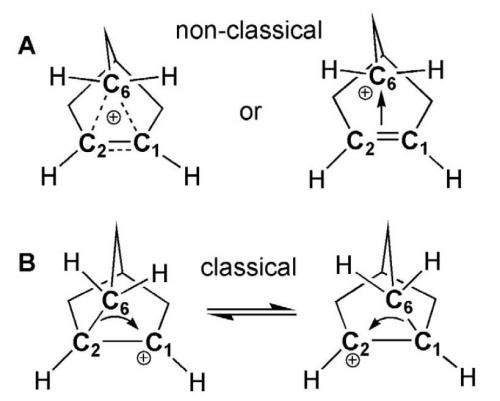
A debate has gone on for 64 years regarding the classical or nonclassical nature of the 2-norbornyl carbocation. It was in 1949 that Saul Winstein suggested their existence to explain the reactivity of substituted norbornane compounds. Other chemists such as Herbert Brown, reacted negatively to the suggestion because it meant accepting that carbon could be bonded to more than four other atoms. Brown suggested instead that a rapid equilibrium could occur to explain what chemists had been observing, which would allow them to remain categorized as classical.
Over the years, the debate has swung back and forth with various chemists arguing for one side or the other, with most eventually leaning towards the nonclassical 2-norbornyl carbocation structure—but now it appears, the chemists in this new effort have finally put the issue to rest. As it turned out, it appeared the vital condition that allowed for settlement of the argument came down to constructing an experiment that involved cooling the crystals with careful annealing at just the right temperature.
The breakthrough came at a lab on the campus of the University of Freiburg. The team used soft bromoaluminate anions to stabilize carbocations in a solid state. That allowed for the preparation of regular 2-norbornyl cation salt crystals. Then, after much work revolving around how the crystals react to cold temperatures, the team arrived at a procedure that involved cooling a sample of the crystal to 40K, then allowing it to warm, then cooling it again—five or six times—doing so finally allowed the crystal structure to reveal itself without cracking in the process.
With the true nonclassical structure of the crystals finally revealed, several chemists have taken the opportunity to pronounce that the results of the work in Germany did little really but prove what most in the field already knew.
More information: Crystal Structure Determination of the Nonclassical 2-Norbornyl Cation, Science 5 July 2013: Vol. 341 no. 6141 pp. 62-64 DOI: 10.1126/science.1238849
Abstract
After decades of vituperative debate over the classical or nonclassical structure of the 2-norbornyl cation, the long-sought x-ray crystallographic proof of the bridged, nonclassical geometry of this prototype carbonium ion in the solvated [C7H11]+[Al2Br7]– • CH2Br2 salt has finally been realized. This achievement required exceptional treatment. Crystals obtained by reacting norbornyl bromide with aluminum tribromide in CH2Br2 undergo a reversible order-disorder phase transition at 86 kelvin due to internal 6,1,2-hydride shifts of the 2-norbornyl cation moiety. Cooling with careful annealing gave a suitably ordered phase. Data collection at 40 kelvin and refinement revealed similar molecular structures of three independent 2-norbornyl cations in the unit cell. All three structures agree very well with quantum chemical calculations at the MP2(FC)/def2-QZVPP level of theory.
Journal information: Science
© 2013 Phys.org