Wound healing: 'See-saw' switch sends cells on the march
Many genes are transcribed into messenger RNA (mRNA) molecules that provide instructions for protein synthesis. Other genes encode regulatory RNAs known as 'microRNAs', which can block protein translation by binding to specific sequences on target mRNAs. Now, researchers led by Prabha Sampath of the A*STAR Institute of Molecular Biology have identified a gene that uses an unusual 'see-saw' mechanism to regulate wound healing by switching between production of mRNA and microRNA.
Sampath and colleagues studied healing with cultured skin cells called keratinocytes, searching for microRNAs that affect the migration of these cells to close newly inflicted wounds. The team focused on miR-198, a microRNA normally produced at high levels but suppressed shortly after injury (see video). Interestingly, miR-198 is derived from the same gene that encodes the FSTL1 protein. The researchers subsequently determined that FSTL1 protein levels rise at the same time as miR-198 levels fall in damaged keratinocyte cultures.
Closer analysis revealed that the microRNA is actually a direct byproduct of the mRNA encoding FSTL1, indicating that cells switch between production of FSTL1 and the microRNA, which is 'edited' from the mRNA. By experimentally reducing keratinocyte production of FSTL1 without affecting miR-198, the researchers inhibited wound healing and identified several healing-associated genes. Forced miR-198 expression had a similar effect on keratinocytes, and inhibited this same set of genes.
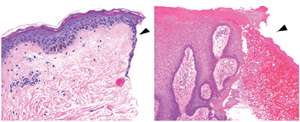
To determine whether these experimental results hold true for human healing, Sampath and co-workers examined patients with chronic non-healing ulcers (see image), a common consequence of diabetes. "Foot ulcers occur in about 15% of diabetic patients, and in 84% of cases they lead to amputation," says Sampath. The team consistently identified high levels of miR-198 and low levels of FSTL1 in cells near the wound edge, indicating an apparently malfunctioning 'switch'.
In many cases, these diabetic ulcers also exhibit defects in a cell signaling pathway triggered by the transforming growth factor β (TGFβ) protein. Sampath and co-workers revealed a direct link between TGFβ activity and FSTL1 production. They showed that TGFβ facilitates expression of the FSTL1 protein and blocks the expression of a protein that promotes miR-198 processing; without TGFβ signaling, miR-198 production prevails and wound healing is blocked.
Previous attempts to treat chronic diabetic ulcers with TGF-β have failed. Having uncovered this TGF-β-modulated FSTL1 switch, however, Sampath now sees a promising way forward for this and other conditions with impaired healing. "We plan to use 'anti-miR-198' molecules to eliminate anti-migratory miR-198," she says. "Coupled with pro-migratory FSTL1 peptides, this may result in effective wound healing."