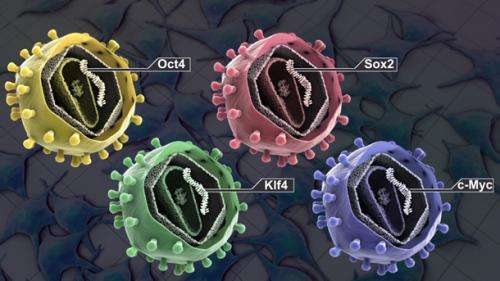
Stem Cell Induction. Credit: stemcellschool.org
(Phys.org) —Gaining control of the ability of mature tissues to generate stem cells is the central medical challenge of our day. From taming cancer, to providing compatible cell banks for replacement organs, knowledge of how cells interconvert between stable points on the complex cellulo-genetic landscape will deliver to the doctor the same mastery the programmer now holds over bits. While researchers often speak of "reprogramming" cells, most recipes today consist only of a crude and partial ingredient list, with little consideration of sequence, quantity or prior state. We recently took stock of the latest in stem cell technology and reviewed the four major factors used to revert adult cells back into omnipotent progenitors. We also just reported on further attempts to rigorously define appropriate level of factors to supply. Researchers from China have now reported that stem cell generation can be regulated by the precise temporal expression of these factors. Publishing in the journal Nature Cell Biology, they show that the efficiency and yield of stem cells can be optimized by controlling the sequencing of the transforming factors, and furthermore provide a theoretical exploration of the possible mechanisms going on behind the scenes.
Back in 2006, researchers in Japan were able to effectively generate stem cells from skin cells without the need for oocytes (eggs) or other embryonic cells. By expressing the four transcription factors, Oct4, Sox2, KLF4 and c-Myc, they could generate cells that, at least in theory, could turn into any other kind of cell. Unfortunately, not only the overall yield of viable stem cells was low, the "rejuvenated" cells that were able to be extracted were generally unsuitable for subsequent patient treatment. The problem is that even when transplanting stem cells obtained from a person's own skin, immune rejection or tumor formation still occurred. This disharmony results from the fact that the immune system, while trained over a lifetime, can be confused by the "dissonance" in expression of youthful protein isoforms, particularly when encountered astride those of more adult cells.
By inducing the expression of all four transformation factors at different times, the Chinese researchers eventually hit upon the optimal sequence. In a nutshell, introducing a combination of Oct4 and Klf4 first, followed later by c-Myc, and then finally Sox2, the maximal yield could be obtained. They were surprised to find that this sequential protocol activated an epithelial-to-mesenchymal (EMT) transition, which was then followed by a delayed reverse (MET) transition. It had been known for some time that in mouse fibroblasts, reprogramming to the pluripotent stem cell state begins by going through a MET conversion. Therefore finding upregulation of the proteins SLUG and N-cadherin, factors generally associated with an EMT, was not anticipated.
In embryogenesis, cells interconvert between epithelial and mesenchymal phenotypes as they lay out the basic body plan. In the epithelial state, cells possess inherent polarity and show preferential adhesion, while in the mesenchymal state, these properties are lost as cells becomes migratory and invasive. This game of run-the bases is recapitulated as more option-constrained cells later rough out the critical form of each organ. Each time cells alights in either camp, they express part of an overlapping subset of various state indicators, but their genetic arrangements are never quite the same.
The authors looked at a few additional factors that might help explain the appearance of a brief mesenchymal state in the sequential procedure. By applying TGF-beta to the simultaneous factor expression protocol early on, they were able to mimic the appearance of the mesenchymal state. This was found to be accompanied by an enhancement in the reprogramming yield, but the effect disappeared when the TGF-beta was applied using a 12-day treatment protocol.
TGF-beta is a whole new can of worms since it is expressed by many cells and does many things, even opposite things in different cells. It is traditionally termed a cytokine, although the distinction between that and a hormone is becoming increasingly blurred. Generally hormones are active at nanomolar concentrations in the blood and vary by less than an order of magnitude. Cytokines by contrast often circulate at less than picomolar concentration and ramp up 1000-fold when called upon during injury or infection.
Capturing the essential behavior of the thousands of downstream regulators or even just four transcription factors, is just not realistic with a flowchart or state diagram. Beyond a certain level of complexity, if the transition probabilities are too low, or the branch points and exceptions too numerous, new constraints are needed before any sensible algorithmic description might be attempted.
In the absence of any such obvious constraints, the authors hypothesized that while multiple pathways exist for conversion between epithelial-mesenchymal states, some are shorter or easier to access than others. They believe that their sequential recipe tips the balance towards a brief mesenchymal state which ultimately leads to a better stem cell yield.
More information: Sequential introduction of reprogramming factors reveals a time-sensitive requirement for individual factors and a sequential EMT–MET mechanism for optimal reprogramming, Nature Cell Biology (2013) doi:10.1038/ncb2765
Abstract
Present practices for reprogramming somatic cells to induced pluripotent stem cells involve simultaneous introduction of reprogramming factors. Here we report that a sequential introduction protocol (Oct4–Klf4 first, then c-Myc and finally Sox2) outperforms the simultaneous one. Surprisingly, the sequential protocol activates an early epithelial-to-mesenchymal transition (EMT) as indicated by the upregulation of Slug and N-cadherin followed by a delayed mesenchymal-to-epithelial transition (MET). An early EMT induced by 1.5-day TGF-β treatment enhances reprogramming with the simultaneous protocol, whereas 12-day treatment blocks reprogramming. Consistent results were obtained when the TGF-β antagonist Repsox was applied in the sequential protocol. These results reveal a time-sensitive role of individual factors for optimal reprogramming and a sequential EMT–MET mechanism at the start of reprogramming. Our studies provide a rationale for further optimizing reprogramming, and introduce the concept of a sequential EMT–MET mechanism for cell fate decision that should be investigated further in other systems, both in vitro and in vivo.
Journal information: Nature Cell Biology
© 2013 Phys.org