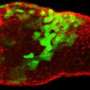
New view of how embryos develop: Imaging technique allows researchers to see how cells form early spinal cord structures
(Phys.org) —In a factory, outside forces dictate the assembly of parts. Robots lift widgets from trays, assemble them and send them on their way. Even though embryos are not factories, theories about embryonic development cast cells as parts to be assembled by outside forces. For instance, according to a longstanding textbook model of neural tube formation, cells sit still and wait for orders, their fates dependent entirely on their locations.
But new research from a team led by Sean Megason, HMS assistant professor of systems biology, gives a dramatically different picture of how cell fates are determined.
Movies of early development show that neural cells decide what fate to adopt while rapidly traveling from place to place, making it hard to see how the textbook model can be true. Megason and colleagues propose that instead of the location telling a cell what identity to adopt, the cell's identity is fixed first and then determines the location.
The work provides insight into how embryos manage to develop the same complex structures despite a wide range of possible environmental and genetic conditions.
"Embryos may be evolutionarily designed to reach the same end in spite of the variation Mother Nature throws their way," said Megason.
These findings were published in the April 25 issue of Cell.
For decades, textbooks have described the formation of the neural tube using a "French flag" model. The flag's stripes correspond to clearly delineated cellular fates that emerge in response to a biochemical signal that spreads across the embryo in a gradient of high to low concentration. The high level concentrations instruct cells to assume one fate, for instance, to become motor neurons. Lower level concentrations mandate different cellular fates.
This orderly model emerged based on snapshots of embryonic development at early and late developmental states.
"In the later stages, everything looks nice," said Megason. "There are sharp stripes of gene expression patterns suggesting that the cell fates were put into place by an outside force."
In the neural tube, that force is a biochemical signal called sonic hedgehog, a developmental gene called a morphogen because it patterns tissue based on its concentration.
Megason, however, has developed a novel imaging and data analysis technique that allows direct observation of the cells in an embryo as they move and change over time. He used it to see what happens in the embryo between the early and late snapshots.
"If you want to understand how something works, a good place to start is to just watch," he said.
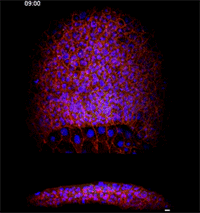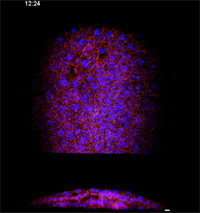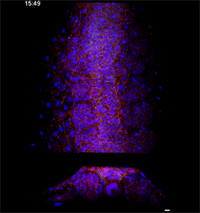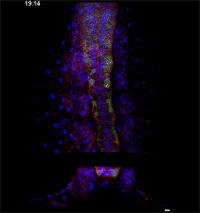
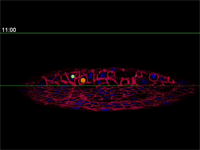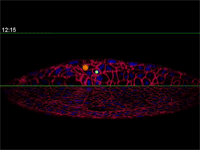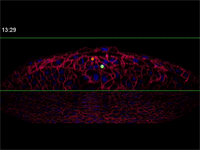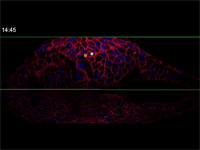
Fengzhu Xiong, first author on the paper and a graduate student in the Megason lab, used the imaging technology to film zebrafish embryos as they grew under a microscope. The embryos had been modified so that every cell was marked and individually identifiable through high-resolution imaging. These markers and software developed in the Megason lab allowed the movements of every cell in the ventral neural tube to be tracked over time.
When the embryo reached a key developmental stage, the cells expressed additional fluorescent labels that indicate each cell's type. The resulting final image reveals sharp lined stripes of cell types that gave rise to the "French flag" model.
By running the video backward, however, Megason and Xiong retraced the steps of every cell, knowing full well each of their fates.
"In the textbook model, the cells should stay in the same place because that model says the cells adopt their fates based on position," Megason said. "But when we tracked the cells back in time, they all got mixed up."
Megason and Xiong found that in response to the morphogen signal, blurry stripes form. The blurring is most likely because the cells don't stay put and, therefore, they don't receive precise orders. Over time, however, the cells sort themselves out into sharp stripes.
The molecular mechanism that governs the sorting of cells is not yet clear. One possibility is that as the cells assume their fates, they turn on different adhesion molecules that make them stick to one another in different ways.
"Once they have these differential affinities, they could self-assemble," said Megason.
Megason is delving deeper into the molecular biology to determine whether adhesion is directing self-assembly. In addition, he is expanding his investigation beyond this study's three cell types, the motor neurons, medial floor plate and lateral floor plate cells, to include more of the dozen or so cell types involved in forming the spinal cord.
Journal information: Cell
Provided by Harvard Medical School