Neighbors move electrons jointly: An ultrafast molecular movie on metal complexes in a crystal
![Sticks and balls model of the transition metal complex iron(II)-tris-bipyridine [Fe(bpy)3]2+. Iron-atoms (Fe) are brown, nitrogen (N) blue, carbon grey, and hydrogen (H) white. The six nitrogen atoms are at the corners of an octahedron around the Fe atom. The planes of the 3 bipyridine subunits (N2C10H8) are mutually perpendicular. Credit: MBI Neighbors move electrons jointly: An ultrafast molecular movie on metal complexes in a crystal](https://scx1.b-cdn.net/csz/news/800a/2013/neighborsmov.png)
Applying femtosecond X-ray methods, researchers at the Max-Born-Institute in Berlin (Germany) and the Ecole Polytechnique Federale de Lausanne (Switzerland) observed an extremely fast, collective electron transfer of ~100 molecular ions after excitation of a single electron in a crystal of transition metal complexes.
Photochemistry and molecular photovoltaics make frequent use of so-called transition metal complexes which consist of a central metal ion bonded to a group of surrounding ligands. Such materials display a strong absorption of ultraviolet or visible light, making them attractive as primary light absorbers in molecular solar cells and other devices of molecular optoelectronics. Absorption of light is followed by an extremely fast shift of electrons from the metal ion to the ligands, a mechanism that is essential for generating an electric voltage. All applications rely on solid state materials in which transition metal complexes are densely packed and can interact with each other. So far, the influence of this interaction on the very fast electron motions following the absorption of light has remained unclear.
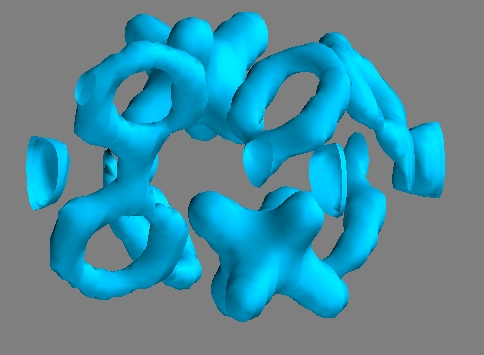
![The counterions in our crystal are two hexa-fluoro-phosphate (PF6-) molecular subunits [phosphorus (P), fluorine (F)]. Again, the six F atoms are at the corners of an octahedron around the P atom. We show here a 3-dimensional surface of constant electron density. The electron density was chosen in such a way that we are most sensitive to the motion of electronic charge located at the PF6- anion. In the movie (www.mbi-berlin.de) we observe upon photo excitation a pronounced reduction of the electron density on that PF6- anion, i.e. a shrinkage of the iso-electron density surface. Credit: MBI Neighbors move electrons jointly: An ultrafast molecular movie on metal complexes in a crystal](https://scx1.b-cdn.net/csz/news/800a/2013/neighborsmov.jpg)
To observe ultrafast electron motions in space and time, one needs to measure the position of electrons in the material with a precision of the order of 0.1 nm (0.1 nm =10-10 m), roughly corresponding to the distance between neighboring atoms, and on a sub-100 fs time scale (1 fs = 10-15s). This is possible by imaging the material with extremely short x-ray pulses which are scattered from the electrons and provide their spatial arrangement. The electron motions are initiated by an ultrashort optical pulse which excites an electron on an individual complex.
In the current issue of Journal of Chemical Physics 138, 144504 (2013) (free download), Benjamin Freyer, Flavio Zamponi, Vincent Juve, Johannes Stingl, Michael Woerner, Thomas Elsaesser and Majed Chergui report the first in-situ x-ray imaging of electron and atom motions induced by such an electron transfer excitation. For the prototype material [Fe(bpy)3]2+(PF6-)2, they show time-dependent 'electron maps' derived from x-ray snapshots taken with 100 fs long hard x-ray flashes. Taking x-ray snapshots at various times during and after the optical pulse that triggers the charge transfer, creates a molecular movie of electron and atom motions.
To the big surprise of the researchers, the time-dependent 'electron maps' reveal a transfer of electronic charge not only from the Fe atoms to the bipyridine units but - so far unknown - an even larger amount of electronic charge from the PF6- counterions to the bipyridine units. The analysis of the x-ray snapshots shows that the charge transfer affects approximately 30 complexes around the directly photo-excited one. This collective electron response is caused by the electric Coulomb forces between the different ions and minimizes the total electrostatic energy in the crystal. Such behavior is highly favorable for charge collection and injection in optoelectronic devices.
More information: Freyer, B. et al. Ultrafast inter-ionic charge transfer of transition-metal complexes mapped by femtosecond X-ray powder diffraction, Journal of Chemical Physics 138, 144504 (2013). jcp.aip.org/resource/1/jcpsa6/ … 44504_s1?bypassSSO=1
Journal information: Journal of Chemical Physics
Provided by Forschungsverbund Berlin e.V. (FVB)


















