When boron butts in: Bridging N–N ligand borylation in group 4 metallocene complexes
For nature and chemists alike, making atmospheric nitrogen available for the formation of more complex nitrogen compounds is both essential and difficult. In the European Journal of Inorganic Chemistry, Paul Chirik and Scott Semproni at Princeton University, USA, report the first examples of the use of group 4 metallocene complexes for boron–nitrogen bond formation from elemental N2.
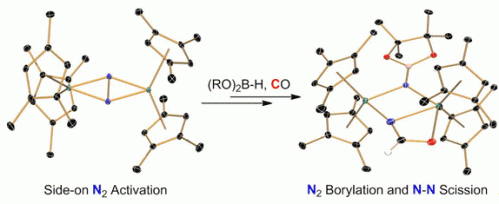
The bond in molecular nitrogen (N2) is very hard to cleave. Transition metal complexes have been used more and more to this end, and recently it was shown that N–N bond cleavage can be coupled to N–element bond formation by use of suitable reagents. Chirik and Semproni achieved this change by treating hafno- and zirconocene complexes containing a highly activated, side-on bound bridging N–N ligand with pinacolborane. Subsequent carbonylation of the borylated fragment leads to N–N bond cleavage and concomitant N–C bond formation.
In contrast, treatment of the borylated metallocene with cyclohexanecarbonitrile or tert-butylisocyanide results only in the insertion of the cyanide ligand into the metal–hydrogen bond but not in cleavage of the N–N bond.
The cyclopentadienyl rings used in the metallocene complexes are well suited for the construction of more elaborate nitrogen-based ligands after dinitrogen functionalization, as they are robust and do not give rise to undesired ancillary ligation. The clean reactivity reported was achieved by a systematic study of the substitution of these ligands to obtain the appropriate activation of the side-on bound N2 ligand. These reactions expand the scope of CO-induced N–N bond cleavage.
More information: Chirik, P. Dinitrogen Borylation with Group 4 Metallocene Complexes, European Journal of Inorganic Chemistry, Permalink to the article: dx.doi.org/10.1002/ejic.201300046
Journal information: European Journal of Inorganic Chemistry
Provided by Wiley