Combining a mixture of sand and paraffin wax produces a more sustainable material for storing heat from the sun for use at night. Credit: American Chemical Society
The search for sustainable new materials to store heat captured from the sun for release during the night has led scientists to a high-tech combination of paraffin wax and sand. Their report on the heat-storing capability of this microencapsulated sand appears in ACS Sustainable Chemistry & Engineering.
Benxia Li and colleagues explain the need for better materials that can store and release heat. These so-called "phase-change" materials" (PCMs) are essential, for instance, for storing heat from the sun for use in providing energy at night or during cloudy periods. PCMs absorb, store and release heat when changing "phases" from a solid to a liquid and vice versa. They have applications that range from expanding use of solar energy to heat-regulating greenhouses to clothing that keeps soldiers or campers warm on cold nights outdoors. Existing PCMs have disadvantages, such as the tendency to leak or catch fire, and Li's team set out to find a better material.
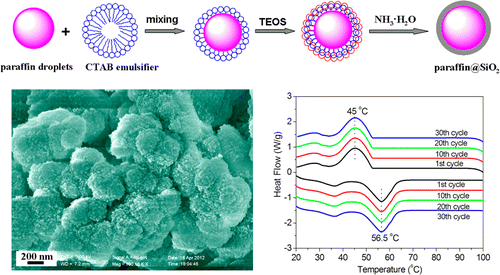
They describe a new approach to using paraffin as a PCM. Made from petroleum, paraffin is a waxy material that absorbs heat, melts into a liquid and releases heat as it solidifies. It involves encapsulating paraffin into tiny spheres of silicon dioxide, the stuff of beach sand. The microencapsulated paraffin has several advantages, including a large surface area that can transfer heat, less reactivity with the environment and less likelihood of leaking as it changes phases. Li's team reports successful tests of the material for 30 melting-solidifying cycles with no leaks at a temperature of 158 degrees Fahrenheit. "The high heat storage capability and good thermal stability of the composite enable it to be a potential material to store thermal energy in practical applications," the report concluded.
More information: "Fabrication and Properties of Microencapsulated Paraffin@SiO2 Phase Change Composite for Thermal Energy Storage" ACS Sustainable Chemistry & Engineering. DOI: 10.1021/sc300082m
Abstract
In this work, a novel microencapsulated phase change composite of paraffin@SiO2 was prepared by in situ emulsion interfacial hydrolysis and polycondensation of tetraethyl orthosilicate (TEOS). The as-prepared paraffin@SiO2 composite was determined by Fourier transformation infrared spectroscope (FT-IR), X-ray diffractometer (XRD), scanning electronic microscope (SEM), and transmission electron microscopy (TEM), respectively. The results showed that the paraffin@SiO2 composite is composed of quasi-spherical particles with diameters of 200–500 nm. The paraffin is encapsulated in a SiO2 shell, and there is no chemical reaction between them. The DSC results indicate that the melting temperature and latent heat of the composite are 56.5 °C and 45.5 J/g, respectively. The encapsulation ratio of paraffin was calculated to be 31.7% from the results of the DSC measurements, slightly lower than the loading content (32.5%) of paraffin in the microencapsulated composite from the TGA measurements. The as-prepared paraffin@SiO2 composite could maintain its phase transition perfectly after 30 melting–freezing cycles, and no leakage of paraffin was observed at 70 °C for 20 min. Moreover, the high heat storage capability and good thermal stability of the composite enable it to be a potential material to store thermal energy in practical applications.
Provided by American Chemical Society