Catalysts that produce 'green' fuel
At the International School for Advanced Studies of Trieste, researchers are studying a way to economically produce a molecule that imitates (and improves) the photosynthesis of plants. It will be used to create solar cells that produce "renewable" fuel respectful of the environment.
The energy produced by solar panels, be it heat or electricity, has to be used right away. It is hard to store and preserve and also its transportation can be rather complicated. Creating solar cells capable of producing energy in an easily storable and transportable way, that is to say fuel, is therefore the future challenge of solar energy. For this reason the scientists at SISSA are working on a catalyst that imitates and improves what nature has been able to do for millions of years.
Plants turn solar energy into sugars, the true "green" fuel, through photosynthesis. In such process a key role is performed by catalysts, molecules that "cut and paste" other molecules, and that in this specific case oxidize water, that is to say separate the hydrogen from the oxygen. Hydrogen (already a fuel itself, yet very hard to handle) is used at a later stage in the synthesis processes that produce sugars from hydrogen and carbon atoms. But scientists are seeking to obtain artificially the same typology of process by using inorganic catalysts, which are faster and more resistant than natural ones (which are very slow: just think of how much time a tree needs to grow). Effective yet costly and limited materials already exist in nature.
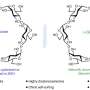
"The crucial part of artificial photosynthesis is water oxidation. We have simulated the way a molecule of Ru4-plyoxometalate (Ru4-POM) functions is this process. Such complex reaction requires catalysts just like the natural process does", explains Simone Piccinin, a researcher of SISSA and of Istituto Officina dei Materiali (CNR-IOM) and lead author of the paper. Ru4-POM was chosen because its effectiveness had been already demonstrated in previous occasions in experiments carried out by the group of ITM-CNR and of Università di Padova that was the first to synthesize the molecule and that has also taken part in this research.
"What was still missing was the comprehension of the process, so we have accordingly reproduced the electronic behavior of the molecule through numeric simulations," underlines Stefano Fabris of SISSA and of CNR-IOM, who has coordinated the theoretical work published in Proceedings of the National Academy of Sciences (PNAS). "We have thus observed that the active sites of the new molecule, that is to say those that convey the reaction, are four atoms of Ruthenium."
"Ruthenium is costly are rare, but now that we know how the atoms that cause the oxidation process have to be arranged we may replace them one by one with cost-effective elements trying to obtain the same level of effectiveness of Ruthenium." concluded Fabris.
Journal information: Proceedings of the National Academy of Sciences
Provided by International School of Advanced Studies (SISSA)