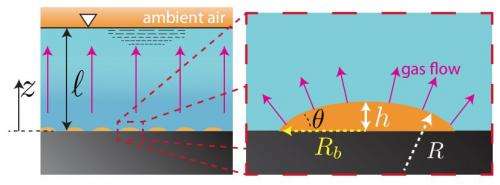
Sketch of a liquid layer in contact with a solid (left). The top of the liquid is exposed to atmospheric conditions. At the solid-liquid interface nanobubbles are present. The arrows indicate the gas flow direction. On the right a further enlargement of one nanobubble is shown. Credit: arxiv.org/abs/1210.3484
(Phys.org)—Physicists in The Netherlands, Detlef Lohse and Joost Weijs of the University of Twente, have offered an explanation of why nano-sized bubbles last considerably longer when sitting on a solid surface covered by a fluid, than when they are allowed float free. In their paper published in Physical Review Letters, the two argue that surface-held bubbles have increased radii of curvature and sit in pools of liquid infused with gas, which causes them to last longer.
Scientists know that the smaller the radii of curvature a bubble has, the less time it will tend to exist before popping. Thus, nano-sized bubbles should pop almost instantly, and in most cases they do. One exception is when they are sitting in a fluid that is covering a solid surface. In this case, nano-sized bubbles have been observed existing for up to days at a time, and until now, no one has been able to offer a reasonable explanation as to why this occurs.
In their paper, the researchers argue that there are two factors at play. The first is the fact that due to the solid surface, the radii of curvature is increased relative to bubbles flowing freely of the same volume, because they are flattened out by the surface. This increases their strength. Second, the researchers noted that the longer lasting bubbles tended to exist in groups of bubbles, rather than as isolated entities. This they means that the liquid in which they all exists has more gas in it (expelled from the bubbles themselves) and thus because it is more saturated, it's less able to accept more gas from the still existing bubbles. Free bubbles on the other hand, leave their gasses behind as they rise to the surface.
Gaining a better understanding of bubble formation and popping is more than a mere curiosity – a more thorough understanding of the underlying principles could lead to better pumps in tiny machines or perhaps assist in the development of medical delivery systems. Also, learning how to prevent bubble formation may also help reduce problems that occur when they impede flow or change the course of fluids in dynamic systems.
More information: Why Surface Nanobubbles Live for Hours, Phys. Rev. Lett. 110, 054501 (2013) link.aps.org/doi/10.1103/PhysRevLett.110.054501 . On ArXiv arxiv.org/abs/1210.3484
Abstract
We present a theoretical model for the experimentally found but counterintuitive exceptionally long lifetime of surface nanobubbles. We can explain why, under normal experimental conditions, surface nanobubbles are stable for many hours or even up to days rather than the expected microseconds. The limited gas diffusion through the water in the far field, the cooperative effect of nanobubble clusters, and the pinned contact line of the nanobubbles lead to the slow dissolution rate.
Journal information: Physical Review Letters
© 2013 Phys.org