Indecisive quanta
(Phys.org)—In ytterbium nickel phosphide there is a quantum critical point between the ferromagnetic and non-magnetic states that was previously not thought possible.
What do the melting points of ice and high-temperature superconductivity have in common? Nothing. Yet there is a peculiar connection. This major unsolved enigma in physics and other quantum mechanical phenomena have to do with what are known as phase transitions, which also includes the melting of ice. However, these are "quantum phase transitions", which are closely intertwined with other quantum mechanical phenomena. They exist entirely at the lower end of the temperature scale, near absolute zero. Physicists from the Max Planck Institute for Chemical Physics of Solids in Dresden have now created an exotic material: At extremely low temperatures, it does not know whether it should pass through a phase transition into a magnetic state or not. It sits at a quantum critical point. Such strange states of matter give clues to help understand exotic phenomena, like high-temperature superconductivity, where a material loses its electrical resistance even at relatively high temperatures.
At present, we experience almost daily snow and ice melting to water, or the opposite, of water freezing to ice. Strictly speaking, such a change in properties is dramatic; in physics it is referred to as a phase transition. A rising temperature generally causes melting first, then vaporisation afterwards, which is true for water as well as for other materials. The molecules in ice occupy ordered positions in the crystal, just like in solids. A supply of thermal energy displaces them from these fixed positions and drives them increasingly into a turbulent motion of molecules: The ice melts into liquid water. Phase transitions are therefore transitions between states having different degrees of order.
Manuel Brando's "Competence Group Extreme Conditions" at the Max Planck Institute for Chemical Physics of Solids in Dresden is also interested in phase transitions. These "quantum phase transitions" are nevertheless as extreme as they are enigmatic. To investigate them, the physicists must cool down their samples to extremely low temperatures. The phenomenon that the Dresden-based researchers are using as a lab mouse, so to speak, is one we are likewise familiar with from everyday life. It is magnetism, in a form that we can even sense, called ferromagnetism more precisely – after the Latin word ferrum, meaning iron. Ferromagnetism is based on a certain form of order as well.
Many exotic phenomena are bound up with a quantum critical point
For iron, the phase transition takes place from an almost non-magnetic state ("paramagnetic") to the ferromagnetic state below 770 degrees Celsius, or 1043 K (K stands for the Kelvin temperature scale). As water freezes to ice, its temperature remains fixed until the water has released so much heat that it becomes completely solidified. This energy-intensive transformation is called a first-order phase transition. In contrast, ferromagnetism develops while cooling without displaying such a halt in temperature change. This smoothly changing temperature behaviour is a hallmark of a second-order phase transition.
The phenomenon that the physicists in Dresden are tracking down also involves second-order phase transitions like this. It is called the quantum critical point and actually exists only at absolute zero. "At a quantum critical point, the curious thing is that it has an effect at much higher temperatures", says PhD student Alexander Steppke. "Many exotic phenomena are associated with it". One of them is the ever enigmatic phenomenon of high-temperature superconductivity which continues to exist at comparatively comfortable, warm temperature ranges up to 135 Kelvin (-138 °C).
A quantum critical point is generally distinguished by the boundary between two different quantum phases disappearing. The material discovered in Dresden can no longer decide whether it wants to be non-magnetic or ferromagnetic at this point. Fundamentally, heat is not allowed to provide the drive for such a quantum phase transition, as it does with water, since such transitions only exist at absolute zero. That is 0 Kelvin or – 273.15 degrees Celsius.
Impurities in the crystal lattice place the material under negative pressure
The scientists in Dresden therefore have to use a different lever, and that is pressure. A mechanical press however, can be ruled out. "Firstly, we require enormous pressures of the order of gigapascals", says Steppke. This is the pressure used in industry to compress carbon into diamond. "Secondly, we also need these pressures in a negative form", explains the physicist. "We need to powerfully expand the samples". That can only happen by using "chemical pressure". To do this, the Dresden-based physicists intentionally build impurities into the crystals of their samples, which increases the volume of the spatial crystal lattice. The trick is not to change the properties of the samples, despite this deliberate contamination.
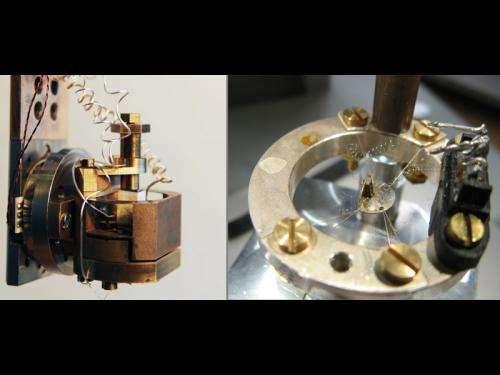
For their experiment, Manuel Brando's group still had to establish a new world record. To do that, their colleague Christoph Geibel and his "Competence Group Materials Development" at the Institute had to design a material that had never existed before. Unlike iron for example, this material only becomes ferromagnetic in the neighbourhood of absolute zero. Thanks to their experience, they were successful, says Brando: "The new ytterbium nickel phosphide material has the lowest Curie temperature that has ever been observed!" This temperature, named after the French Nobel Physics laureate Pierre Curie, describes the point at which the phase transition to ferromagnetism takes place. Yet what creates the magnetism?
Spins can still rotate at absolute zero
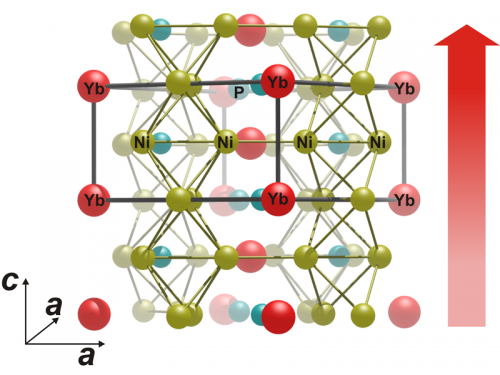
Magnetic ytterbium atoms within the crystal are responsible for this. Electrons sitting within such atoms behave like tiny magnetic elements that can rotate. The "spins", which produce their micromagnetism, feel each other. In ferromagnets, all these spins are aligned in one direction and their collective order produces bulk magnetism. In ytterbium nickel phosphide (YbNi4P2), this ferromagnetic transition lies so close to absolute zero that an additional famous quantum effect also comes into play: the Heisenberg uncertainty principle. Actually, no phase transition whatsoever should be possible at this extreme cold where thermal energy is non-existent, and any kind of movement should be frozen. The electron spins should therefore no longer be able to switch back and forth between ferromagnetism and non-magnetic disorder. "Due to the uncertainty principle, however, their energy is not precisely determined", says Steppke, "and therefore they really can still rotate".
With a sophisticated experiment, the scientists were able, for the first time, to observe a quantum critical point during transition between a ferromagnetic and non-magnetic state in a metal; standard theories had excluded its existence and must now be corrected. The basic researchers in Dresden hope they can contribute to solving the riddle of high-temperature superconductivity with these experiments.
More information: Steppke, A. et al. Ferromagnetic Quantum Critical Point in the Heavy-Fermion Metal YbNi4(P1−x Asx)2, Science, 22 February 2013, DOI: 0.1126/science.1230583
Journal information: Science
Provided by Max Planck Society