January 21, 2013 report
Materials scientists create highly water repellant ceramics (w/ video)
(Phys.org)—Researchers at MIT have created several new types of ceramics that all demonstrate a high degree of liquid repellency. All are based, they write in their paper published in the journal Nature Materials, on the oxides of the lanthanides, and unlike most ceramics are extremely hydrophobic.
Scientists are interested in creating materials that repel liquids, because clinging droplets can lead to problems with machinery, such as inefficiencies in steam turbines, or in other applications, e.g. ice developing on airplane wings. Most attack the problem by applying a coat of hydrophobic material, but that is a stop-gap measure since most such materials tend to wear away quickly. The new ceramics developed at MIT represent a material that can be used as a base product, rather than as a coating.
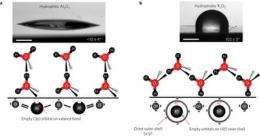
The researchers note that the reason water sticks to a material is because electrons in the water's oxygen atoms are shared with atoms from the material to which it sticks (or vice-versa), causing bonding to occur. To get around that, they looked to the metals that sit near the bottom of the Periodic Table – the oxides of the lanthanides (cerium, lutetium, etc.). Such elements have empty oribitals surrounded by a shell of electrons, making them less conducive to sharing. They created small discs of ceramic material using each of the metals (except radioactive promethium, of course) as a base and then tested them all by dumping water droplets on them.
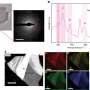
The team found that every one of the discs repelled water. They also noted that when water was allowed to condense on the surface of the discs, it did so as droplets, rather than the messy splashes seen with hydrophilic materials. They next subjected the discs to harsh conditions to see how robust they were and found that one in particular, based on cerium oxide, was able to withstand grinding and temperatures up to 1,000 °C – afterwards it continued to show its natural repellency.
The next step for the team is to begin creating machine parts out of the ceramic materials and to test them in real world conditions to see if they are able to withstand the rigors of everyday wear and tear.
More information: Hydrophobicity of rare-earth oxide ceramics, Nature Materials (2013) doi:10.1038/nmat3545
Abstract
Hydrophobic materials that are robust to harsh environments are needed in a broad range of applications1, 2, 3. Although durable materials such as metals and ceramics, which are generally hydrophilic, can be rendered hydrophobic by polymeric modifiers4, these deteriorate in harsh environments. Here we show that a class of ceramics comprising the entire lanthanide oxide series, ranging from ceria to lutecia, is intrinsically hydrophobic. We attribute their hydrophobicity to their unique electronic structure, which inhibits hydrogen bonding with interfacial water molecules. We also show with surface-energy measurements that polar interactions are minimized at these surfaces and with Fourier transform infrared/grazing-angle attenuated total reflection that interfacial water molecules are oriented in the hydrophobic hydration structure. Moreover, we demonstrate that these ceramic materials promote dropwise condensation, repel impinging water droplets, and sustain hydrophobicity even after exposure to harsh environments. Rare-earth oxide ceramics should find widespread applicability as robust hydrophobic surfaces.
Journal information: Nature Materials
© 2013 Phys.org