January 4, 2013 weblog
Researchers force a gas to a temperature below absolute zero
(Phys.org)—A team of physicists in Germany have succeeded in forcing a gas to become colder than absolute zero. Using lasers and a magnetic field to manipulate an ultra-cold gas, the researchers, as they describe in their paper published in the journal Science, managed to coax the temperature of the gas to a few billionths of a Kelvin below absolute zero.
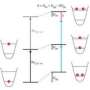
Absolute zero was first defined by Lord Kelvin back in the mid 1880s, as the lowest possible temperature state, where atoms stop moving. The temperature scale bearing his name starts at that lowest point, but over the past several decades, scientists have discovered that there are exceptions to the rule and that at least theoretically, it should be possible for a system to produce conditions where temperatures fall lower than absolute zero. This is possible, they say, because the temperature of a system is generally considered to be the average energies of the particles in it. Most hover around a certain point, with a few moving to higher levels. But, when the system is turned upside down, with most of the particles exhibiting higher energy levels, and just a few have lower energy, the system is reversed as are the temperature signs, indicating temperatures below absolute zero.
To turn such a system upside down in the real world, the physicists started by chilling a quantum gas made up of potassium atoms to near absolute zero. They used lasers and magnetic fields to force the atoms into a lattice pattern. At temperatures above absolute zero, the atoms naturally want to repel one another, keeping the system stable. But by adjusting the lasers and magnetic field, the researchers were able to force the atoms to attract one another, essentially, turning the system on its head. At positive temperatures, they note, such a system would quite naturally be unstable – to force it to be stable, the team also adjusted the lasers that held the atoms trapped in place. Doing so, they report, resulted in the gas transitioning to a temperature below absolute zero.
While the achievement won't likely result in the creation of such systems for practical purposes, it does help better understand the principle of temperature, and may, some suggest, help explain other still mysterious phenomenon, such as why the universe is continuing to expand, despite the pull of gravity – which some have attributed to a force called dark energy. There are parallels, the researchers say, between the way the gases in a sub-absolute zero system want to collapse, but don't due to the negative absolute temperatures and the negative force preventing the universe from doing the same.
More information: Negative Absolute Temperature for Motional Degrees of Freedom, Science, 4 January 2013: Vol. 339 no. 6115 pp. 52-55 DOI: 10.1126/science.1227831
ABSTRACT
Absolute temperature is usually bound to be positive. Under special conditions, however, negative temperatures—in which high-energy states are more occupied than low-energy states—are also possible. Such states have been demonstrated in localized systems with finite, discrete spectra. Here, we prepared a negative temperature state for motional degrees of freedom. By tailoring the Bose-Hubbard Hamiltonian, we created an attractively interacting ensemble of ultracold bosons at negative temperature that is stable against collapse for arbitrary atom numbers. The quasimomentum distribution develops sharp peaks at the upper band edge, revealing thermal equilibrium and bosonic coherence over several lattice sites. Negative temperatures imply negative pressures and open up new parameter regimes for cold atoms, enabling fundamentally new many-body states.
Journal information: Science
© 2013 Phys.org