Clear view into glass: Researchers have analysed the atomic structure of amorphous silica
We can look through glass, but what glass itself looks like on the inside has so far remained a mystery - at least as far as the precise position of the atoms is concerned. Scientists at the Fritz-Haber-Institute of the Max Planck Society in Berlin are now the first to have imaged the network of silicon and oxygen atoms - the main components of glass - in a silica film. They used two methods that image individual atoms in surfaces to analyse the glass film, which is a mere two atomic layers thick. Being able to see the atomic structure enabled the researchers to confirm that glass is structured as the Norwegian-American physicist William H. Zachariasen predicted back in 1932. Moreover, in further studies, the researchers observed the transition from a crystalline to a disordered - scientists call it amorphous - two-dimensional structure. Their findings could assist the semiconductor industry, for example, to produce amorphous silica in a more controlled way, and should also facilitate the search for new, more powerful catalysts.
The semiconductor industry likes to have complete control over its production - and it can now work with one unknown less. Studies of glass undertaken by researchers in the group headed by Markus Heyde and Hans-Joachim Freund at the Fritz Haber Institute of the Max Planck Society have now provided several insights into the atomic structure of an important raw material used in the chip industry, which uses amorphous silica as an insulator in every transistor. "Previously, we knew nothing about what happens here," says Markus Heyde. "It is generally quite astonishing how little is known about glass, which is so important in nature, in our daily life and for many technical applications." Therefore, if scientists now ascertain the precise structure of this material class, it could assist not least the semiconductor industry to improve the processing of amorphous silica.
However, the findings could also be useful in the search for new, powerful catalysts, which reduce the energy required for a chemical reaction, steer it in the desired direction or even make it possible at all. Disordered silica often serves as the substrate of the actual catalyst and affects its properties; one additional good reason to find out more about the substrate material. And that is exactly what the researchers in Berlin have done.
The first chance to check an 80-year-old proposal for the structure
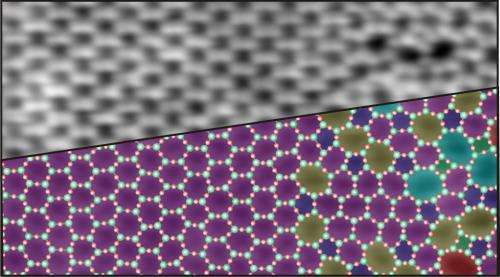
"This is the first time we have been able to directly observe which characteristic elements the structure possesses and which patterns occur in it," says Markus Heyde. According to the observations, silicon and oxygen atoms take their turns in the individual silica layers and form a network of rings that lie next to each other like soap bubbles floating on water, and also assume sizes which differ almost as much - starting with rectangular rings with only four atoms through to those with nine or more atoms. Hexagonal rings are the most frequent, and the rings become all the rarer the further the number of their atoms deviates from six. William H. Zachariasen had already proposed such a structure for amorphous silica 80 years ago. This structure has each silicon atom in a plane surrounded by three oxygen atoms, just like in crystalline silica. However, crystalline silica forms a regular honeycomb structure that is composed solely of hexagons in a plane.
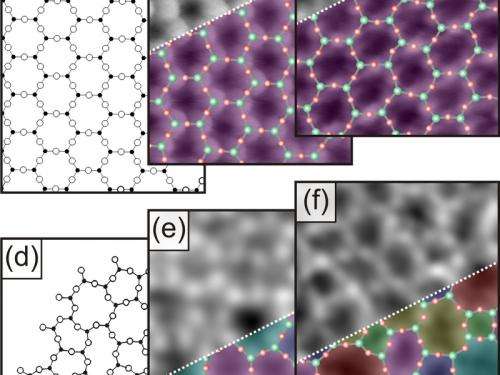
It had so far not been possible to check the structure proposed by William Zachariasen, as X-ray diffraction, the method of choice to determine the structure of materials, cannot be used in glasses and in amorphous materials in general - at least, not for determining the precise positions of the atoms. The Berlin-based researchers succeeded in doing just this with a trick; however, they designed a two-dimensional model of a glass. In an ultrahigh vacuum chamber they produced just two atomic layers of silica on a substrate of the precious metal ruthenium, which they had earlier coated with an oxygen layer.
The completely planar structure allows the scientists to look at the structure
"Depending on how we change the temperature during the sample preparation, and how much silicon and oxygen we release in the vacuum, we obtain an amorphous or a crystalline structure, or even a mixture of both," explains Leonid Lichtenstein, who investigated the different structures in his doctoral thesis. This allowed the researchers to produce silica surfaces which are so smooth that even the glass mirrors of the best telescopes in the world, which are polished to an extremely high level of precision, would look like hilly the Rocky Mountains by comparison.
"The structure of the two-dimensional glass provides us with important information about the structure of a three-dimensional glass," says Markus Heyde. This is primarily due to the fact that the completely planar glass film allows researchers to view its very own structure for the first time. In planar surfaces the positions of the individual atoms can be determined, even if the structure is amorphous.
In a preliminary study, the scientists scanned amorphous silica with a scanning tunnelling microscope, which tells them where the oxygen atoms are located. This already revealed the irregular meshes of the network which William Zachariasen proposed as the structure of glass. In two further studies, the researchers also scanned the surfaces of their samples with a non-contact atomic force microscope which can also detect the silicon atoms. They thus obtained an accurate image of all atomic positions in the two-dimensional glass.
Knowing the glass structure helps to develop catalysts
The scientists also investigated the transition between an amorphous and a crystalline silica film. "We determined that, apart from the hexagons, it is mainly pentagonal and heptagonal shapes that occur at the boundary initially - i.e. the most similar rings," says Markus Heyde. The further they moved their microscope's gaze from the crystalline to the amorphous region, the more the ring sizes deviated from the crystalline hexagonal structure.
Now that the researchers have a crystal-clear view of each individual atom in an amorphous silica film, they want to investigate how the different structural elements behave when foreign atoms or molecules end up on the glass surface. Do they attach preferentially to rings of a specific size? "We are interested in this question because such absorption processes are important for heterogeneous catalysis," explains Hans-Joachim Freund, director at the Fritz Haber Institute. The researchers also want to answer this question in order to clarify whether their two-dimensional silica is also suitable as a model substance for a catalyst substrate which is actually in use; its two atomic layers form a network of connected cages with openings of different widths, which could serve as a type of atomic sieve. It is also possible that different elements can be stored selectively in these cage-like structures.
More information: Lichtenstein, L. et al., Crystalline-Vitreous Interface in Two Dimensional Silica. Phys. Rev. Lett., 2012. prl.aps.org/abstract/PRL/v109/i10/e106101
Lichtenstein, L. et al., Atomic Arrangement in Two-Dimensional Silica: From Crystalline to Vitreous Structures. J. Phys. Chem. C., 2012. pubs.acs.org/doi/abs/10.1021/jp3062866
Lichtenstein, L. et al., The Atomic Structure of a Metal-Supported Vitreous Thin Silica Film. Angew. Chem. Int. Ed., 2012. onlinelibrary.wiley.com/doi/10 … e.201107097/abstract
Journal information: Physical Review Letters , Angewandte Chemie International Edition
Provided by Max Planck Society












.jpg)