Imaging technique helps scientists profile the sugar structures on protein molecules
The body's cell machinery often attaches sugar chains onto proteins to create molecules that play a crucial role in everything from protective immunity to structural stability. However, distinguishing between the different sugar tags applied to these so-called 'glycoproteins' can be difficult. Now, investigators in Japan have developed a way to visualize the profile of sugar molecules (known as 'glycans') on specific protein targets.
The new method could help scientists investigate the expression and trafficking of glycoprotein targets with therapeutic potential. For example, recent studies have revealed that changes in glycan structures accompany the progress of disease. "Our technique may help understand why this is happening," says Tadashi Suzuki of the RIKEN Advanced Science Institute in Wako.
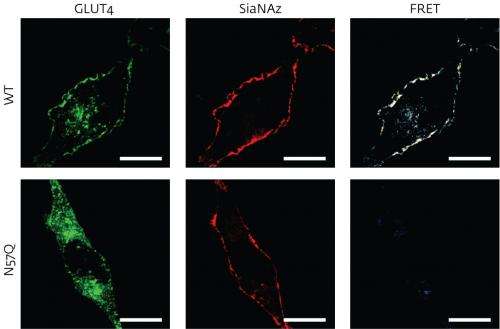
To determine the nature of attached sugars, Suzuki and his postdoctoral fellow Yoshimi Haga turned to an imaging technique known as 'fluorescence resonance energy transfer', or FRET. The researchers labeled sugar molecules with a fluorescent probe that is activated only when signals are emitted from a nearby protein tagged with green fluorescent protein (GFP). These signals are picked up by the FRET assay, which can reveal the complex spatial and geometrical characteristics of the glycoprotein structures, even those that span the thick cell membrane.
As a proof of principle, Suzuki and Haga first applied the method to two well-studied glycoproteins—the glucose transporter GLUT4 and the epidermal growth factor receptor—each tagged with a specific type of sugar tag, sialic acid. The researchers then set up an experiment in human cell culture where they adjusted the levels of insulin. This hormone triggers the transport of GLUT4 to the cell surface, where the glycoprotein begins pumping glucose into the cell. The scientists found that lowering insulin levels led to GLUT4 proteins with sugars containing sialic acid re-entering the cell more slowly than GLUT4 proteins without this kind of sugar adornment. "We do not know why it happens," Suzuki says. "This finding may shed some light on the cell trafficking of closely related glycoproteins."
The researchers now want to adapt the technique so they can visualize sugars on more complex glycoproteins. "GLUT4 happens to be fairly easy as it only contains a single glycan," Suzuki says, "but for proteins bearing multiple glycans, it is not going to be so easy, as the structural diversity is much greater."
More information: Haga, Y. et al., Visualizing specific protein glycoforms by transmembrane fluorescence resonance energy transfer. Nature Communications 3, 907 (2012). www.nature.com/ncomms/journal/ … full/ncomms1906.html
Journal information: Nature Communications
Provided by RIKEN