Virus exploitscellular waste disposal system
ETH Zurich researchers demonstrate how vaccinia virus manipulates the cellular waste-disposal system and thereby cleverly tricks the cell into assisting the intruders replication. Now, the virologists have turned the tables, using inhibitors of this cellular waste-disposal system as a way to block virus infection.
Over the years, researchers in the laboratory of ETH-professor Ari Helenius have elucidated the tricks and tactics viruses use to enter human cells and exploit them for their own multiplication and spread. Jason Mercer, senior researcher in the Helenius lab at the Institute of Biochemistry is quite excited about his latest publication. In a collaboration with Berend Snijder and colleagues from the Universtiy of Zürich they have just released a publication which puts forward new insights into how viruses enter human cells.
"For the first time we were able to demonstrate a mechanism by which a virus uses the cellular waste-disposal system to facilitate release of the viral DNA, which is subsequently multiplied, and used for the formation of new virus particles" he says. In addition, the researchers were able to block the release of viral DNA – using a drug which is already approved for human use.
Complete protein inventory
During infection, viruses communicate with the host cell and they "abuse" a specific set of host proteins to assist them during their life-cycle. In collaboration with the group of University Professor Lukas Pelkmans, Jason Mercer set out to identify the cellular proteins which the vaccinia virus requires. The idea being that this knowledge may be helpful when developing new strategies to stop infection.
This virus serves as a well studied model system. Vaccinia virus was used as a vaccine for the eradication of smallpox. To identify these proteins, the researchers used an RNA interference screening-procedure which allowed them to test thousands of cellular factors for a possible requirement during infection.
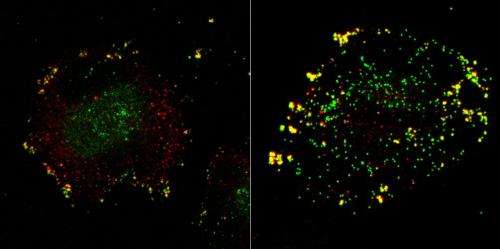
The technology allows researchers to silence single human genes in order to test if, and at which stage, they might be involved in the infection process. After screening 7,000 candidates, the researchers identified 188 cell factors that contribute to sucessful infection.
Tricking the waste-disposal system
Thereafter, to analyze these proteins the researchers first clustered them into groups based on their physical interactions and cellular functions. One functional group was particularly prominent: Components of the cells waste disposal system. On one hand, this cellular machinery serves to transfer a small molecule called ubiquitin to other proteins. Ubiquitin is a molecular waste-label which targets proteins for degradation. On the other hand the disposal system contains a molecular-shredder, the so-called proteasome, which destroys these "labelled" proteins.
The DNA genome of vaccinia virus is surrounded by a shell made of more than 40 different proteins which is referred to as the viral core. To the researchers surprise, they found that the proteins which make up the core of the virus are heavily ubiquitinated during virus assembly. When a vaccinia virus enters a new cell, this ubiquitin label brings the proteasome to the scene, which cuts the core proteins into small pieces thereby leading to the release of the virus` genetic information.
The finding that vaccinia virus uses the proteasome-ubiquitin system to release the viral DNA is novel. "Vaccinia virus steals the cells waste tags and uses them for its own purposes" Mercer explains. The cell cannot just get rid of its proteasomes because waste-disposal is an essential process for the cell. Without it, the cell would be flooded with cellular trash.
Inhibiting the protein shredder
The american researcher and first author of a recent Cell Reports publication points out that the latest technological advances have made it possible to test so many different genes at once. "High-throughput screening has lead to novel insights into the fields of cell biology and virus infection" he emphasizes.
This screen can serve as a starting point for the discovery of compounds which inhibit cellular proteins required for infection. When the researchers blocked the proteasome using an inhibitor, the viral cores could no longer be degraded. "The proteasome inhibitor is very efficient. Upon treatment, the DNA cannot get out of the virus particle." Mercer stresses. Other researchers have shown that proteasomes are needed for virus replication as well. In this way, blocking proteasome function presents the virus with a number of unsoluable challenges. Neither can the DNA be freed nor can the virus start to replicate is genome. Ultimately, vaccinia virus is rendered unable to generate progeny.
Cancer-drug could be used as virotherapy
Proteasome inhibition is nothing new. To treat multiple myeloma, a malign cancer of the bone, the drug Velcade is currently being used. Velcade (also called bortezomib) occupies the active sites of the proteasome thereby inactivating the shredder. This inhibition causes the cancer cells to undergo cell death. Similarly, could Velcade potentially be used to block virus infections that depend on the activity of proteasomes? Admittedly, the drug has strong side effects as the cellular waste disposal is rendered dysfunctional. Nevertheless, Dr. Mercer thinks it may be conceivable to use Velcade as a basis of antiviral therapy.
"Temporary inactivation of proteasomes could be a general strategy to fight poxviruses and other DNA viruses which rely on proteasome function, such as herpes viruses" Mercer says. Since proteasome inhibition targets a cellular function and not vaccinia virus directly, the virus may have a hard time adjusting to this. Therefore he thinks it unlikely that the virus develops resistance against a potential antiviral proteasome inhibitor.
More information: Mercer J, et al. RNAi screening reveals proteasome- and Cullin3-dependent stages in vaccinia virus infection. Cell Report, published online 18th October 2012. DOI:10.1016/j.celrep.2012.09.003
Provided by ETH Zurich