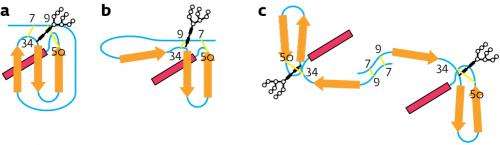
Structures of the properly folded glycoprotein: a) a misfolded isomer, b) the dimer, and c) substrates for UGGT. Disulfide linkages are highlighted in yellow. Credit: 2012 Yasuhiro Kajihara, Osaka University and Yukishige Ito, RIKEN Advanced Science Institute
The endoplasmic reticulum of cells provides a pivotal quality-control system that eliminates improperly folded, or misfolded, glycoproteins, such as antibodies and hormones. The UDP-glucose:glycoprotein glycotransferase (UGGT) enzyme is central to this system: it binds to incompletely folded proteins and facilitates biochemical reactions that lead to their proper folding. However, the rules governing UGGT's reactivity remain unclear. Now, a synthetic approach is available for biochemists to relate this reactivity with protein folding. The method, which produces a series of intentionally misfolded glycoproteins to probe UGGT's selectivity, was developed by a team led by Yasuhiro Kajihara of Osaka University, working with the Japan Science and Technology Agency's ERATO Glycotrilogy Project, directed by Yukishige Ito of the RIKEN Advanced Science Institute, Wako, Japan.
Previous investigations relied on denatured glycoproteins obtained from natural sources, but the carbohydrate portion of these compounds usually displayed heterogeneous structures and compositions, making UGGT's properties difficult to assess precisely.
Kajihara, Ito and colleagues synthesized a homogeneous, artificial glycoprotein using a protein known as interleukin-8, which has a well-understood architecture. Interleukin-8 contains sulfur-based functional groups that form so-called disulfide linkages, allowing the researchers to introduce folds at desired positions either within or between protein strands and generate structures with varying folded geometries, or isomers.
To obtain the model glycoprotein, the researchers divided interleukin-8 into two segments and grafted a carbohydrate pendant to one of the segments before joining the resulting molecules together. In the presence of cysteine and cystine amino acids, the glycoprotein folded correctly. The absence of cysteine and cystine produced three supplementary misfolded isomers: two structures containing two disulfide linkages and a dimer. The dimer consisted of two strands, bearing one intramolecular disulfide, which were bridged by two additional intermolecular disulfides.
Evaluation of UGGT's reactivity revealed that the enzyme was active only when incubated with misfolded glycoproteins. Furthermore, the dimer was the most reactive isomer. Improper folding exposes water-repelling patches on protein surfaces, leading to speculation that UGGT's specificity may originate from this hydrophobicity. "Our analyses unambiguously demonstrated that the isomer with the highest surface hydrophobicity, namely the dimer, also exhibited the highest activity, matching this hypothesis," says Ito.
"We are now eager to uncover the mechanistic details involving UGGT, especially how this enzyme deals with a variety of glycoproteins," he adds. The team is also interested in reconstituting the folding process at the laboratory level. "Complete understanding of this process will be beneficial in the production of bioactive and medicinally important glycoproteins," says Ito.
More information: Izumi, M., Makimura, Y., Dedola, S., Seko, A., Kanamori, A., Sakono, M., Ito, Y. & Kajihara, Y. Chemical synthesis of intentionally misfolded homogeneous glycoprotein: a unique approach for the study of glycoprotein quality control. Journal of the American Chemical Society 134, 7238–7241 (2012). pubs.acs.org/doi/abs/10.1021/ja3013177
Journal information: Journal of the American Chemical Society
Provided by RIKEN