Credit: Uon
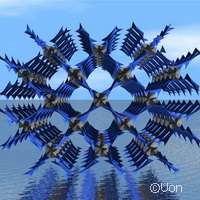
(Phys.org)—Researchers at the University of Nottingham in the United Kingdom recently discovered a novel material that could be used by sophisticated technologies to fight global warming. The study was funded in part by an European Research Council (ERC) Advanced Grant worth EUR 2.5 million awarded to Professor Martin Schröder for the COORDSPACE project ('Chemistry of coordination space: extraction, storage, activation and catalysis') [under the EU's Seventh Framework Programme (FP7)]. The results, recently presented in the journal Nature Chemistry, demonstrate that this material, called NOTT-300, could substitute for carbon dioxide (CO2) absorption.
'Our novel material has potential for applications in carbon capture technologies to reduce CO2 emissions and therefore contribute to the reduction of greenhouse gases in the atmosphere,' said research leader Prof. Martin Schröder of the University of Nottingham. 'It offers the opportunity for the development of an "easy on/easy off" capture system that carries fewer economic and environmental penalties than existing technologies. It could also find application in gas separation processes where the removal of CO2 or acidic gases such as SO2 is required.
According to the researchers, their findings could help us understand how to solve the problem of greenhouse gases. 'It is widely accepted that it is imperative that the CO2 footprint of human activity is reduced in order to limit the negative effects of global climate change,' Prof. Schröder said. 'There are powerful drivers to develop efficient strategies to remove CO2 using alternative materials that simultaneously have high adsorption capacity, high selectivity for CO2 and high rates of regeneration at an economically viable cost.'
The researchers found that NOTT-300 covers all these criteria. Thanks to its properties, NOTT-300 could boost environmental and chemical sustainability. With regards to cost, this material is synthesised from relatively simple and inexpensive organic materials (we would say "product" instead of "materials" but please only change if correct scientifically. The only solvent is water.
'The material shows high uptake of CO2 and SO2,' the Nottingham researcher said. 'In the case of SO2, this is the highest reported for the class of materials to date. It is also selective for these gases, with other gases - such as hydrogen, methane, nitrogen, oxygen - showing no or very little adsorption into the pores.'
Additionally, the team found that the material facilitates the release of absorbed gas molecules through pressure loss, and it has high chemical stability to all common organic solvents. NOTT-300 is also stable in water and is resistant to high temperatures up to 400 °C.
More information: Yang, S., et al. 'Selectivity and direct visualization of carbon dioxide and sulfur dioxide in a decorated porous host', Nature Chemistry, 2012. doi:10.1038/nchem.1457
Journal information: Nature Chemistry
Provided by CORDIS