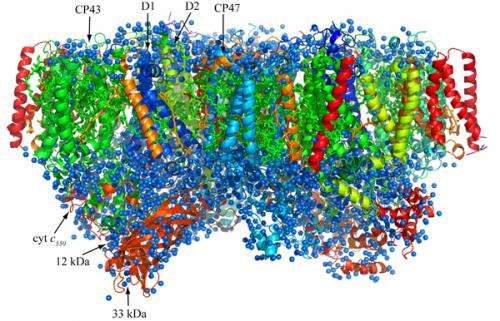
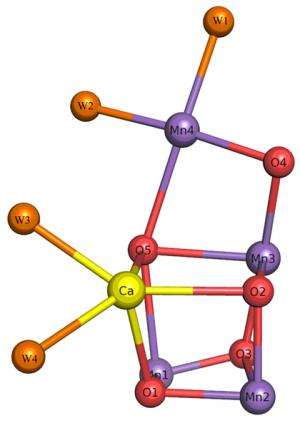
Overall structure of photosystem II.
Oxygenic photosynthetic organisms utilize energy from the sun to split water into protons, electrons and oxygen—products vital to life on earth. The process takes place through light-induced electron transfer reactions in a membrane protein complex photosystem II, but so far the resolution of structural studies on the protein complex has been too limited to ascertain the mechanism of these reactions in detail.
Now Jian-Ren Shen at Okayama University in collaboration with researchers at Osaka City University in Japan has solved the structure of the photosystem II complex at an unprecedented resolution. They improved the quality of the photosystem II crystals significantly, and obtained X-ray diffraction data with a resolution of 1.9 Å.
Structure of the chair-shaped oxygen-evolving complex.
Their studies revealed the detailed structures of amino acid residues and a number of cofactors involved in light absorption, energy transfer, and electron transfer reactions in this protein complex. The most significant finding of their work is elucidation of the detailed structure of the Mn4CaO5 cluster, which catalyses the light-induced water-splitting reaction. The cluster is shaped like a distorted chair and the distances between atoms in the structure provide insights into the role of oxygen and nearby water molecules in dioxygen formation. As they pointed out, "This provides a basis for unravelling the mechanism of water splitting and O–O bond formation, one of nature's most fascinating and important reactions." Their studies are considered extremely helpful for artificial photosynthesis that aims to derive clean energy from the sun light efficiently, which may provide an ultimate solution for the energy and environmental problems that we face.
More information: Yasufumi Umena, et al. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473, 55-61 (2011). DOI: 10.1038/nature09913
Journal information: Nature
Provided by Okayama University