Interfaces are key in metal oxide superlattices
(Phys.org)—Materials called transition metal oxides have physicists intrigued by their potentially useful properties—from magnetoresistance (the reason a hard drive can write memory) to superconductivity.
By combining two sophisticated experimental tools—oxide molecular beam expitaxy and angle-resolved photoemission spectroscopy—researchers have gained the first insights into quantum interactions in transition metal oxide superlattices, which are artificial stacked layers of alternating materials, each just a few atoms thick.
Even slight modifications to the stacking sequence can switch the entire superlattice from a conductive to insulating state, due to the enhancement of quantum interactions between the electrons. The findings were published online Aug. 19 in the journal Nature Materials.
"We are interested in superlattices of transition metal oxides because they can exhibit all sorts of exotic electronic and magnetic properties that do not exist in the bulk of these materials," said Kyle Shen, assistant professor of physics and paper's senior author. "They might be useful someday, but from a scientific standpoint, they are just really fascinating because the electrons can conspire to give rise to very unexpected emergent phenomena."
For some transition metal oxide superlattices, it has been shown that adding just one extra layer of atoms to the stacked layers switches them from conductor to insulator. Shen and his colleagues wanted to understand why this occurs.
To do this, the team tapped the expertise of co-author Darrell Schlom, the Herbert Fisk Johnson Professor of Industrial Chemistry in the Department of Materials Science and Engineering, who with postdoctoral scholar Carolina Adamo, created specifically designed stacks of two oxides, lanthanum manganese oxide and strontium manganese oxide, each just a few atomic layers thick and with atomic precision. To make the superlattices, they used molecular beam epitaxy, which is like spray-painting with the elements of the periodic table.
The team then utilized a unique piece of instrumentation designed and built by Shen and Schlom's groups at Cornell. It allowed them to study the superlattices after synthesis by angle-resolved photoemission spectroscopy without exposing the surfaces to air, which would contaminate the sample and obscure the sensitive experiments. Eric Monkman, a graduate student in Shen's group, and colleagues then measured and analyzed how the electrons move through different kinds of superlattices.
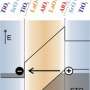
It turned out that the distances between the interfaces of the lanthanum and strontium oxides were the key: Pushing the interfaces farther apart made the electrons more confined to each individual interface, resulting in an enhancement of the quantum interactions, which drive the entire superlattice into an insulating state.
By pushing the interfaces closer together, the electrons could start to move between interfaces, resulting in a metallic state. The researchers were able to reach these conclusions through the use of photoemission spectroscopy, which maps the motion of electrons in solids at the atomic scale.
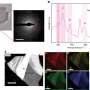
Advanced transmission electron microscopy imaging led by David A. Muller, Cornell professor of applied and engineering physics and co-director of the Kavli Institute at Cornell for Nanoscale Science, and graduate student Julia Mundy, confirmed that the interfaces between the lanthanum and strontium were indeed sharp, which helped confirm the quantum interactions.
The paper's co-first authors are Monkman and Adamo. Shen, Schlom and Muller are members of the Kavli Institute at Cornell for Nanoscale Science. The research was supported by the National Science Foundation through the Cornell Center for Materials Research and a Career award.
Journal information: Nature Materials
Provided by Cornell University