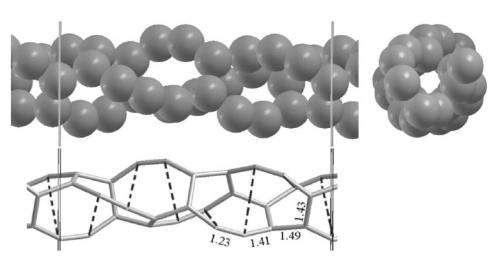
(Top) Front and lateral view of the new 3.2-Å-thick CNT10R nanotube. Vertical lines indicate the unit cell. (Bottom) Stick view of the structure, with broken bonds indicated by dashed lines (bond distances in Å units). Image credit: Menéndez-Proupin, et al. ©2012 American Physical Society
(Phys.org)—Carbon nanotubes (CNTs) are renowned for their thinness, having diameters as small as 3 angstroms (Å), or 0.3 nm. It's generally thought that ultrathin CNTs with diameters smaller than 3 Å are unstable because, at that scale, the bonds that hold the atoms together become distorted and lead to collapse. So far, the thinnest of these CNTs – those thinner than 4 Å – have been found only confined inside a thicker CNT. In a new study, scientists have presented simulations that show that a CNT with an outer diameter of just 3.2 Å can theoretically exist without confinement and remain stable at temperatures up to 1000 K, which would make it one of the thinnest CNTs ever synthesized.
The scientists, Eduardo Menéndez-Proupin of the Autonomous University of Madrid and the University of Chile; Ana L. Montero-Alejo of the Autonomous University of Madrid and the University of Havana; and José M. García de la Vega of the Autonomous University of Madrid, have published their study in a recent issue of Physical Review Letters.
"There are reports of CNTs 3 angstroms thin contained inside a thicker CNT," Menéndez-Proupin told Phys.org. "Our CNT may be the thinnest one capable of existing freestanding."
As the scientists explain, the ultrathin CNT that they examined results from the relaxation – or bond-breaking – of a CNT made from a graphene sheet that is cut and wrapped a certain way, as defined by its chirality. In this case, the original CNT has chirality (2,1), a diameter of 2 Å, and is unstable.
By breaking certain bonds of this particular CNT, the researchers theoretically showed that the resulting structure becomes stable in vacuum, forming a 3.2-Å-thick, non-standard CNT. Due to the broken bonds, the new CNT consists of rings that are each made of 8 and 10 atoms. Consequently, the researchers named this structure CNT10R, after the 10-atom rings.
The simulations revealed that the 10-atom rings along with smaller rings form a double helix, similar to the structure of DNA, with alternating single, double, and triple bonds. Using Quantum ESPRESSO software, the scientists calculated the new nanotube's optical and electronic properties, which differ significantly from those of standard CNTs, nanoribbons, and graphene sheets. Instead, CNT10R's properties resemble those of linear carbon chains, suggesting that the structure can be thought of as a pair of twinned chains.
"All known nanotubes have the form of a honeycomb lattice (graphene) rolled, and all the atoms are trifold coordinated," Menéndez-Proupin said. "The smallest rings are 6-membered. The structures have vacancies and other defects, but this is the minority of atoms and in general represent an increment of energy. The CNT10R does not have 6-membered rings, is periodic and stable. It displays all kinds of bonds. The IR and Raman spectra are quite different from the standard CNTs and graphene. The triple bond is not frequent in carbon structures. The presence of triple bonds could facilitate specific chemical reactions that are not possible in other CNTs."
Knowing these properties could help researchers experimentally find or synthesize the new structure in the future. One possible route for synthesis may involve growing the structure inside a larger CNT, which may be more technically feasible than growing a freestanding one, for now.
"Synthesis may be possible with existing technology, although this would not be freestanding," Menéndez-Proupin said. "It may have been synthesized by accident, but it has not been identified. It could be identified in existing samples if a tubular structure is observed by microscopy and the same structure produces a weird spectrum that looks similar to our predictions."
More information: E. Menéndez-Proupin, et al. "Ultrathin Carbon Nanotube With Single, Double, and Triple Bonds." PRL 109, 105501 (2012). DOI: 10.1103/PhysRevLett.109.105501
Journal information: Physical Review Letters
Copyright 2012 Phys.org
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.