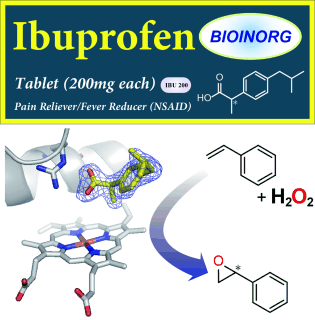
Pain-reliever ibuprofen makes enzyme oxidize styrene with hydrogen peroxide
Enzymes can be efficient biocatalysts for organic synthesis, but often only work on a small range of specific substrates. Yoshihito Watanabe and colleagues at Nagoya University and RIKEN (Japan) have tricked enzymes into catalyzing reactions between molecules of choice, they report in Chemistry—An Asian Journal. By using organic acids, including the pain reliever ibuprofen, they mimicked the natural substrate.
The researchers used one member of the family of so-called cytochrome P450 enzymes, which owe their catalytic activity to the same heme group that binds oxygen in hemoglobin in the blood. They were able to expand its substrate scope by using organic acids to simulate substrate binding. The carboxylic acid group activates the H2O2 oxidant, which finally results in oxidation of the heme iron atom and generation of the active species.
This "decoy" approach allows epoxidation of styrene, which cannot normally be achieved with P450 enzymes. When an asymmetric decoy is used, such as the common pain reliever ibuprofen, the epoxidation proceeds stereoselectively: Only the desired molecule is produced, but not its mirror image, which can make the difference between a drug and a poison.
Further optimization of the system both in terms of decoy molecule and P450 mutant should yield higher catalytic activity and better stereoselectivity. This method should prove valuable in expanding the use of enzymes in organic synthesis.
More information: by Yoshihito Watanabe, Chiral-Substrate-Assisted Stereoselective Epoxidation Catalyzed by H2O2-Dependent Cytochrome P450SPα, Chemistry - An Asian Journal, Permalink to the article: dx.doi.org/10.1002/asia.201200250
Journal information: Chemistry - An Asian Journal
Provided by Wiley