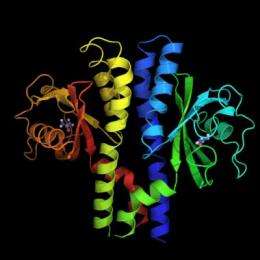
H. pylori's acid receptor TlpB is seen to the left and right side of the bacterium. This outward-reaching receptor helps H. pylori navigate away from high levels of stomach acid. Credit: Courtesy S. James Remington
University of Oregon scientists have discovered how the bacterium Helicobacter pylori navigates through the acidic stomach, opening up new possibilities to inactivate its disease-causing ability without using current strategies that often fail or are discontinued because of side effects.
Their report -- online ahead of regular publication July 3 in the journal Structure -- unveils the crystal structure of H. pylori's acid receptor TlpB. The receptor has an external protrusion, identified as a PAS domain, bound by a small molecule called urea and is poised to sense the external environment. TlpB is the first bacterial chemoreceptor of known function shown by crystallography to contain an extracellular PAS domain, the researchers reported.
"It is a beautiful structure, and this domain has never been seen before in this class of proteins," said co-author S. James Remington, professor of physics and member of the UO Institute of Molecular Biology. Captured at the atomic resolution of 1.38 angstroms, it is the first new, significant structural view in 20 years of the class of receptors used by bacteria to navigate their chemical environment.
H. pylori, a Gram-negative bacterium, was first identified in 1982 and shown to be associated with stomach ulcers and stomach cancer. While its mode of transmission is not precisely understood, the bacterium is found in the stomach of half of the people in the world, said co-author Karen Guillemin, professor of biology and also a member of the Institute of Molecular Biology. To fight H. pylori infections, patients generally are treated with broad-spectrum antibiotics, but the bacterium is becoming resistant and treatment fails in about 30 percent of cases.
As part of the new UO study -- led by postdoctoral researcher Emily G. Sweeney and doctoral student J. Nathan Henderson, now a postdoctoral researcher at Arizona State University -- researchers manipulated the atomic structure of the protein to disrupt its ability to bind urea, and demonstrated that the urea is the key to how the bacterium senses and avoids acid.
When the receptor is unable to bind urea, Guillemin said, the bacteria became confused and were unable to navigate away from high acid. "We found that this urea binding is absolutely crucial for this protein to act as an acid sensor," she said.
"We now have significant new insights into how acid sensing works at the atomic level which is important for H. pylori's life in the stomach" Guillemin said. "The health implications are this: If we disrupt the binding of urea, we can confuse the bacteria and potentially block their ability to reach the stomach lining where they cause damage."
The new research so far only presents "snapshots along the way" of the H. pylori's signaling process, Remington said. More study is needed to understand the mechanisms involved, but the new structure now lets researchers see the some 3,000 individual atoms -- and where they are located -- in the newly discovered urea-binding protein domain.
"This interdisciplinary research team worked together to approach a problem and provide fundamental new knowledge about this bacterium's basic structure," said Kimberly Andrews Espy, vice president for research and innovation at the UO. "As a result, we are able to understand the discovery in the context of disease and how human health might benefit."
Journal information: Structure
Provided by University of Oregon