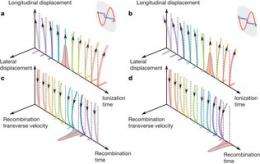
Schematic description of the two-colour gates. Image (c) Nature 485, 343-346 (17 May 2012) doi:10.1038/nature11025
(Phys.org) -- In a bit of inspired research, a diverse team of researchers has devised a means for measuring the time it takes for an electron to tunnel through a barrier. Led by Israel's Weizmann Institute of Science, Dror Shafir, the team as they describe in their paper published in the journal Nature used one laser to lower a barrier allowing an electron to escape via tunneling from its Helium atom, and another to prod it back again and in the process were able to measure the time it took to do so.
Quantum tunneling is where particles are able to move through a barrier even though they lack sufficient energy to do so. It’s also a process that happens at such speed that it’s been almost impossible to measure. Making things even more difficult is the fact that measurement of the speed of tunneling is described differently depending on whether it is inside or outside of its barrier. Outside, the speed at which a particle moves is described by normal Newtonian physics. Inside of its barrier however, it’s described by a complex number, which is calculated by combining a real and imaginary number. Making things even murkier is the fact that particles such as electrons don’t exist in a single state, but rather as a quantum wave, which is described by a wavefunction.
To calculate the tunneling time of an electron escaping the barrier imposed by the interaction between it and the nucleus of its Helium atom, the research team focused a strong laser field on the electrons of a single Helium atom, causing the barrier holding them in place to lower just enough to allow them to escape the barrier by tunneling. The team then used a second less powerful laser field to push the electrons back to the ion that had been created, causing them to reconnect and return the barrier to a normal state. When each did so, a single photon with higher energy than the initial laser field was released that could be seen by the team. As a result, they were able to measure the time it took for the whole process to occur (just attoseconds) and found that it matched quantum theory, proving that they’d succeeded in their efforts.
The team also conducted another experiment where they forced electrons from carbon dioxide molecules and compared the times for ionization between those that were taken from a highest energy level and those from two levels that were deeper, which takes more energy. They found that the difference between the two (time to tunnel) was close to 40 attoseconds.
Taken together, the results obtained by the team demonstrate that tunneling can not only be timed, but it can be done in multiple ways.
More information: Resolving the time when an electron exits a tunnelling barrier, Nature 485, 343–346 (17 May 2012) doi:10.1038/nature11025
Abstract
The tunnelling of a particle through a barrier is one of the most fundamental and ubiquitous quantum processes. When induced by an intense laser field, electron tunnelling from atoms and molecules initiates a broad range of phenomena such as the generation of attosecond pulses1, laser-induced electron diffraction and holography. These processes evolve on the attosecond timescale (1 attosecond ≡ 1 as = 10−18 seconds) and are well suited to the investigation of a general issue much debated since the early days of quantum mechanics—the link between the tunnelling of an electron through a barrier and its dynamics outside the barrier. Previous experiments have measured tunnelling rates with attosecond time resolution and tunnelling delay times. Here we study laser-induced tunnelling by using a weak probe field to steer the tunnelled electron in the lateral direction and then monitor the effect on the attosecond light bursts emitted when the liberated electron re-encounters the parent ion. We show that this approach allows us to measure the time at which the electron exits from the tunnelling barrier. We demonstrate the high sensitivity of the measurement by detecting subtle delays in ionization times from two orbitals of a carbon dioxide molecule. Measurement of the tunnelling process is essential for all attosecond experiments where strong-field ionization initiates ultrafast dynamics10. Our approach provides a general tool for time-resolving multi-electron rearrangements in atoms and molecules—one of the key challenges in ultrafast science.
Journal information: Nature
© 2012 Phys.Org