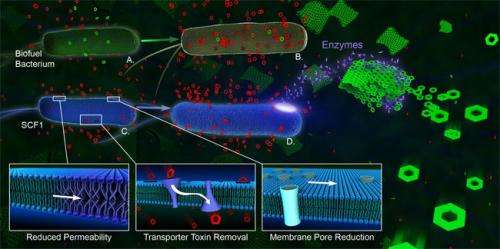
Different bacterial responses to ionic liquid. In (A), a fermentative bacterium is exposed to sugars for biofuel synthesis along with an ionic liquid (IL) used during the production process. The ionic liquid exerts a toxic effect soon after it enters the cell (B). In contrast, the rainforest isolate E. lignolyticus in (C) can tolerate relatively high levels of the ionic liquid, even as it secretes enzymes (D) that degrade plant cellulosic material to glucose and other sugars. A mechanistic model for bacterial resistance to ionic liquid includes: reducing membrane permeability to ionic liquid by remodeling the phopholipid bilayer (introduction of kinked fatty acids in lower left inset); removing intracellular ionic liquid through membrane transport proteins (efflux pumps shown as purple cones in lower middle inset); and, slowing the influx of ionic liquid by decreasing the number of porins (transmembrane cylinders in lower right inset). Green objects: glucose (rings) and cellulosic materials (sheets); red objects: ionic liquid; purple objects: enzymes.
(Phys.org) -- Using new experimental methods and computational analysis, a team of scientists from the Joint BioEnergy Institute (JBEI), led by Lawrence Livermore's Michael Thelen, discovered how certain bacteria can tolerate manmade toxic chemicals used in making biofuels.
By deciphering the makeup of a bacterium found in the soil of a tropical rain forest, scientists may have a better understanding of how to more efficiently produce biofuels.
The production of liquid fuels derived from plant biomass offers a promising technology for reducing greenhouse gas emissions and dependence on fossil fuels.
While sugars stored within the plant cell wall, known as lignocellulose, are plentiful enough to supply most energy needs on the planet, their extraction is difficult and requires chemical pretreatment followed by enzymatic digestion using micro-organisms. However, while ionic liquids – salty solvents – improve the digestibility of lignocellulose, they also are toxic to bacteria used in subsequent conversion steps.
Using new experimental methods and computational analysis, a team of scientists from the Joint BioEnergy Institute (JBEI), led by Lawrence Livermore's Michael Thelen, discovered how certain bacteria can tolerate manmade toxic chemicals used in making biofuels.
"Discovering microbes naturally tolerant to salty liquids and understanding their mechanisms of tolerance should significantly enhance biofuel production," Thelen said.
The team describes an in-depth characterization of native bacterial resistance to the toxic effects of an ionic solvent used in biofuel production in the May 14 online edition of the journal, the Proceedings of the Natural Academy of Sciences (PNAS).
Microbes found in natural environments, such as decomposing forest soils, produce highly efficient enzymes to degrade lignocellulose and are often able to adapt to stressful changes in the environment. An alternative approach involves harnessing the positive traits of these native bacteria to engineer existing laboratory strains for more effective biofuel processing in the presence of toxic solvents.
Thelen and his colleagues isolated Enterobacter lignolyticus strain SCF1, a bacterium that can degrade lignocellulose from plant biomass. They found that SCF1 grows well in the presence of relatively high concentrations of an ionic liquid that is used for lignocellulose pretreatment. This chemical is highly toxic to other bacterial species.
The El Yunque National Forest in Puerto Rico is a tropical rainforest where a strain of the microbe Enterobacter lignolyticus was found that can tolerate an ionic liquid used to dissolve cellulosic biomass for microbial-based biofuel production. Credit: (Photo by Kristen DeAngelis)
"We looked into how SCF1 tolerates this ionic liquid by using high-throughput growth assays, composition analysis of the bacterial cell membrane, and by determining all of the genes that are differentially expressed when SCF1 cells are exposed to an ionic liquid," Thelen said.
By mapping these analyses onto all of the known metabolic reactions identified in the genome sequence of SCF1, the team found that the bacteria can resist the toxic effects of an ionic liquid by adjusting the composition of membranes to reduce cell permeability, and by increasing a type of transport protein that can pump the toxic chemical out of the cell before damage occurs.
"Vigorous efforts to discover and analyze micro-organisms with properties similar to those of SCF1 have the potential to greatly benefit industrial processes," Thelen said. "These results will be used as a foundation for genetic engineering of ionic liquid-tolerant microorganisms for a more efficient biofuel production process."
Provided by Lawrence Livermore National Laboratory