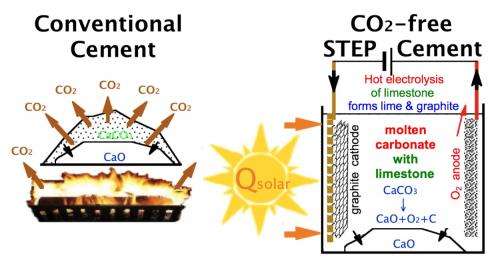
In the conventional production of lime from limestone, fossil fuels are burned during the decarbonation process, resulting in a carbon dioxide byproduct. In the STEP process, solar thermal energy is used to heat the limestone as well as assist in electrolysis, producing a different chemical reaction with no carbon dioxide byproduct. Image credit: Licht, et al. ©2012 The Royal Society of Chemistry
(Phys.org) -- While the largest contributor to anthropogenic greenhouse gas emissions is the power industry, the second largest is the more often overlooked cement industry, which accounts for 5-6% of all anthropogenic CO2 emissions. For every 10 kg of cement produced, the cement industry releases a full 9 kg of CO2. Since the world consumes about 3 trillion kg of cement annually, this sector has one of the highest potentials for CO2 emission reductions. But while processes are being explored to sequester the CO2 from cement production, so far no process can completely eliminate it.
Jumping on this opportunity for improvement, a team of researchers from George Washington University in Ashburn, Virginia, has developed a method for cement production that releases zero CO2 emissions. In addition, the scientists estimate that the new production process will be cheaper than the existing process used in the cement industry.
In their study published in a recent issue of Chemical Communications, the scientists describe the process as the Solar Thermal Electrochemical Production of cement, or STEP cement. (The team previously used a similar STEP process for carbon capture, with the potential for decreasing CO2 levels in the atmosphere to pre-industrial levels.)
As the scientists explain, 60-70% of CO2 emissions during cement production occurs during the conversion of limestone into lime. This conversion involves decarbonation, or removing the carbon atom and two oxygen atoms in limestone (CaCO3) to obtain lime (CaO) with CO2 as the byproduct. The remainder of the emissions comes from burning fossil fuels, such as coal, to heat the kiln reactors that produce the heat required for this decarbonation process.
The STEP process addresses both issues, starting by replacing the fossil fuel heat source with solar thermal energy. The solar heat is not only applied directly to melt the limestone, it also provides heat to assist in the electrolysis of the limestone. In electrolysis, a current applied to the limestone changes the chemical reaction so that instead of separating into lime and CO2, the limestone separates into lime and some other combination of carbon and oxygen atoms, depending on the temperature of the reaction. When electrolyzed below 800°C, the molten limestone forms lime, C, and O2. When electrolyzed above 800°C, the product is lime, CO, and ½O2.
“Electrolysis changes the product of the reaction of the limestone as it is converted to lime,” coauthor Stuart Licht, a chemistry professor at George Washington University, told Phys.org. “Rather than producing carbon dioxide, it reduces the carbon dioxide (adds electrons) and produces only oxygen and graphite (which can be readily stored as solid carbon) or CO for fuels, plastics or pharmaceuticals. This is accomplished at low energy and high throughput.”
When separated, the carbon and oxygen atoms no longer pose the threat to the atmosphere that they do as CO2. As Licht explained, the carbon monoxide byproduct in the higher temperature reaction can be used in other industries, such as to produce fuels, purify nickel, and form plastics and other hydrocarbons. Plus, the carbon monoxide is produced significantly below market value by this solar thermal electrolytic process. The main product, lime, doesn't react with the other byproducts, but instead forms a slurry at the bottom of the vessel where it can easily be removed.
“This study presents a low-energy, entirely new synthetic route to form CaO without any carbon dioxide emission, and is based on unexpected solubility behavior in molten salts,” Licht said. “This synthesis can be accomplished without solar energy, and without our new STEP process, but is particularly attractive when combined with this new solar process. Alternatively, the new synthesis could be used by industry to produce cement using any non-solar renewable or nuclear energy without any CO2 release, or greatly decrease CO2 if fossil fuels were used to drive the new cement production (in the latter, worst-case scenario, the products are lime, graphite and oxygen; there is still no CO2 product, but CO2 would be used in the energy to drive the process).”
According to the researchers, the STEP process can be performed at a lower projected cost than the existing cement industry process. In fact, when accounting for the value of the carbon monoxide byproduct, the cost of the lime production is actually negative. The researchers' rough analysis shows that the total cost of the limestone material, solar heat, and electricity is $173 per ton of lime and 0.786 tons of carbon monoxide (0.786 tons of carbon monoxide are produced for every ton of lime). The market value of carbon monoxide is $600 per ton, or $471 per 0.786 tons. So after selling the carbon monoxide, the cost of the lime production is $173 - $471 = -$298 per ton. For comparison, the cost to produce lime in the conventional way is about $70 per ton. The researchers emphasize that this analysis is not comprehensive, but it indicates the cost benefit of STEP cement, not even considering the value of eliminating CO2 emissions.
The scientists add that the STEP process could be extended beyond cement production to other applications that convert limestone to lime, such as purifying iron and aluminum; producing glass, paper, sugar, and agriculture; cleaning smoke stacks; softening water; and removing phosphates from sewage.
The next challenge for the researchers lies in scaling up the process for commercialization. They note that Gemasolar, a large-scale solar thermal plant, is already in operation. Other solar thermal plants are following, with electricity costs expected to decrease. To maintain constant operation, molten salt storage of the thermal energy can allow production to continue even during fluctuations in sunlight and at night. Another issue may be finding enough lithium carbonate for the electrolyte, although the metal is not consumed in the STEP process and so is not a recurring cost.
“We plan to scale up the outdoor STEP cement prototype, and in general want to increase the portfolio of useful chemicals made by our new solar process,” Licht said. “The goals are to replace today's fossil fuel economy with a renewable chemical economy. Scale-up is the challenge. Although the process is entirely new, the individual components (solar towers, 24/7 operation storing solar energy with molten salts) are already in place. Solar energy can be used to efficiently make products without carbon dioxide, and at solar energy efficiencies higher than in photovoltaics.”
More information: Stuart Licht, et al. “STEP Cement: Solar Thermal Electrochemical Production of CaO without CO2 emission.” Chem. Commun., DOI: 10.1039/C2CC31341C
© 2012 Phys.Org