That caffeine in your drink -- is it really 'natural?'
That caffeine in your tea, energy drink or other beverage — is it really natural? Scientists are reporting successful use for the first time of a simpler and faster method for answering that question. Their report appears in the American Chemical Society (ACS) journal Analytical Chemistry.
Maik A. Jochmann, Ph.D., and colleagues point to the growing consumer preference for foods and beverages that contain only natural ingredients. Coffee, tea, colas, energy drinks and other caffeine-containing drinks are the most popular beverages in the world. Food regulatory agencies require that caffeine be listed on package labels, but do not require an indication of whether the caffeine is from natural or synthetic sources. The scientists set out to develop a faster, simpler method for categorizing caffeine's origins.
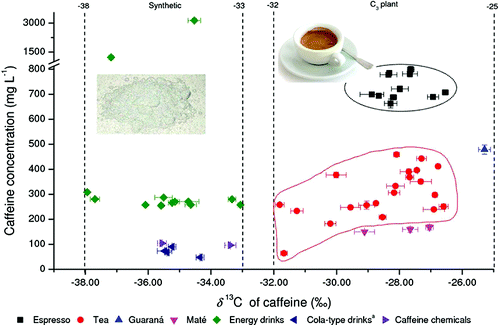
In the study, they describe use of a technique called stable-isotope analysis to differentiate between natural and synthetic caffeine. The test makes use of differences in the kinds of carbon isotopes – slight variations of the same element – found in caffeine made by plants and caffeine made in labs with petroleum-derived molecular building blocks. Their analysis, which takes as little as 15 minutes, found four products that contained synthetic caffeine, despite a "natural" label.
More information: Caffeine in Your Drink: Natural or Synthetic? Anal. Chem., Article ASAP. DOI: 10.1021/ac203197d
Abstract
Owing to possible adulteration and health concerns, it is important to discriminate between natural and synthetic food ingredients. A new method for compound-specific isotope analysis (CSIA) by coupling high-temperature reversed-phase liquid chromatography to isotope ratio mass spectrometry (HT-RPLC/IRMS) was developed for discrimination of natural and synthetic caffeine contained in all types of drinks. The analytical parameters such as stationary phase, column inner diameter, and column temperature were optimized for the separation of caffeine directly from drinks (without extraction). On the basis of the carbon isotope analysis of 42 natural caffeine samples including coffee beans, tea leaves, guaraná powder, and maté leaves, and 20 synthetic caffeine samples from different sources by high-temperature reversed-phase liquid chromatography coupled to isotope ratio mass spectrometry, it is concluded that there are two distinguishable groups of caffeine δ13C-values: one between −25 and −32‰ for natural caffeine, and the other between −33 and −38‰ for synthetic caffeine. Isotope analysis by HT-RPLC/IRMS has been applied to identify the caffeine source in 38 drinks. Four mislabeled products were detected due to added but nonlabeled synthetic caffeine with δ13C-values lower than −33‰. This work is the first application of HT-RPLC/IRMS to real-world food samples, which showed several advantages: simple sample preparation (only dilution), high throughput, long-term column stability, and high precision of δ13C-value. Thus, HT-RPLC/IRMS can be a very promising tool in stable isotope analysis of nonvolatile compounds.
Provided by American Chemical Society