February 29, 2012 feature
Rational design can improve hydrogen fuel cell efficiency
(PhysOrg.com) -- Hydrogen fuel cells, in which the chemical energy of hydrogen is converted into electricity, offer the potential for a wide variety of applications, especially in transportation and power generation. Although hydrogen fuel cells are currently used on a small scale, making them commercially available for large-scale use requires improvements in two key areas: efficiency and cost-effectiveness. In a new study, scientists have designed tri-metallic electrocatalysts for hydrogen fuel cells that theoretically improve in both areas, outperforming the best platinum-based catalysts to date.
The researchers, Sergey Stolbov and Marisol Alcántara Ortigoza from the University of Central Florida, have published their study on the new efficient electrocatalysts in a recent issue of The Journal of Physical Chemistry Letters.
Currently, most hydrogen fuel cells use catalysts made of platinum, a rare and expensive material. Finding an alternative to platinum is challenging because few materials can withstand exposure to the fuel cells’ highly acidic solvents, which dissolve most transition metals. Only four elements – platinum, iridium, gold, and palladium – can resist corrosion, but none is ideal. Platinum and iridium are rare and expensive, while gold and palladium do not perform well due to low redox reactivity.
In this study, Stolbov and Alcántara Ortigoza focused on improving the redox reactivity of gold and palladium through the use of in-depth modeling.
“We have proposed a new concept for rational design of stable and highly active electrocatalysts for hydrogen fuel cells,” Stolbov told PhysOrg.com. “We believe that our approach is much more efficient than the widely used combinatorial screening of dozens of materials. Our first attempt to apply this approach has resulted in the prediction of two cost-effective and highly active catalysts for hydrogen fuel cells, a clean and renewable energy source.”
The researchers explained that previous attempts at searching for better catalyst designs have used trial and error, although some studies have used computational searches. By using a rational design approach, the researchers could predict the performance of different tri-metallic catalyst designs using previous knowledge, such as the relationship between the composition/morphology and electronic structure of the catalyst surface, its stability, the thermodynamics that the reaction intermediates, and the reaction kinetics.
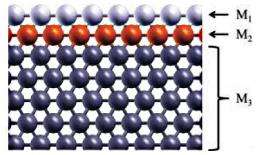
This method led the researchers to a new design consisting of a three-layered sandwich-like structure. In this design, redox reactions take place on the first layer, while the second layer can tune the electronic structure of the first layer, and the third layer serves as the substrate. As an example, the researchers used gold as the first layer, then chose ruthenium as the second layer due to its ability to tune the gold layer to increase its redox reactivity. When using palladium as the first layer, their method predicted iron as a good tuning material. The ruthenium and iron do not have to come in contact with the acidic solvent, yet still contribute to the catalyst’s efficiency. In both cases, the researchers used tungsten as the substrate, which also contributed to performance in a twice-removed way by straining the middle layer.
The researchers’ calculations showed that both the gold-based and palladium-based sandwich-like catalysts were more highly reactive and therefore more efficient than today’s best platinum-based catalysts. How much more efficient can only be accurately determined by experiments, which the researchers hope will be performed soon.
“We are looking forward to experimental confirmation of our findings,” Stolbov said. “We are contacting experimentalists who are interested in these materials. Using our approach, we have already selected a number of sandwich-like structures as promising electrocatalysts in an attempt to find even better catalysts. In order to test this rational selection, we will perform computational studies of the electronic structure and stability of the chosen systems and the thermodynamic properties of the reaction intermediates on the catalyst surfaces. Then again, we will ask experimentalists for testing the most promising systems. Since our approach can be extended to design the stable structures tuned for reactions in heterogeneous catalysts, we will focus on this subject next.”
More information: Sergey Stolbov and Marisol Alcántara Ortigoza. “Rational Design of Competitive Electrocatalysts for Hydrogen Fuel Cells.” The Journal of Physical Chemistry Letters, 2012, 3, 463-467. DOI: 10.1021/jz201551e
Copyright 2012 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.



















