Children may be receiving the highest exposure to nanoparticles of titanium dioxide in candy, which they eat in amounts much larger than adults, according to a new study. Published in ACS' journal, Environmental Science & Technology, it provides the first broadly based information on amounts of the nanomaterial – a source of concern with regard to its potential health and environmental effects – in a wide range of consumer goods.
In the study, Paul Westerhoff, Ph.D., and colleagues point out that titanium dioxide is a common additive to many consumer products, from food to paint to cosmetics. Westerhoff explained that the body releases the nanoparticles in feces and urine, sending them to wastewater treatment plants, which cannot prevent the smallest particles from entering lakes and rivers. Only one previous study, done a decade ago, reported on titanium dioxide content in a few commercial products. To fill the knowledge gap about the sources of humans' exposures, the researchers bought and tested food, personal care products, paints and adhesives and measured how much titanium dioxide they contain.
The group found that children consume more titanium dioxide than adults because sweets like candies, marshmallows and icing are among the products with the highest levels. The paper lists the names of the products tested and their titanium dioxide content. Westerhoff recommends that regulators shift their focus from the type of titanium dioxide used in paints and industrial processes to food-grade particles, because those are much more likely to enter the environment and pose a potential risk to humans and animals.
More information: Titanium Dioxide Nanoparticles in Food and Personal Care Products, Environ. Sci. Technol., Article ASAP. DOI: 10.1021/es204168d
Abstract
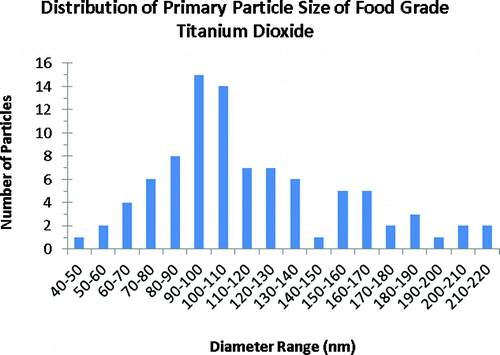
Titanium dioxide is a common additive in many food, personal care, and other consumer products used by people, which after use can enter the sewage system and, subsequently, enter the environment as treated effluent discharged to surface waters or biosolids applied to agricultural land, incinerated wastes, or landfill solids. This study quantifies the amount of titanium in common food products, derives estimates of human exposure to dietary (nano-) TiO2, and discusses the impact of the nanoscale fraction of TiO2 entering the environment. The foods with the highest content of TiO2 included candies, sweets, and chewing gums. Among personal care products, toothpastes and select sunscreens contained 1% to >10% titanium by weight. While some other crèmes contained titanium, despite being colored white, most shampoos, deodorants, and shaving creams contained the lowest levels of titanium (<0.01 μg/mg). For several high-consumption pharmaceuticals, the titanium content ranged from below the instrument detection limit (0.0001 μg Ti/mg) to a high of 0.014 μg Ti/mg. Electron microscopy and stability testing of food-grade TiO2 (E171) suggests that approximately 36% of the particles are less than 100 nm in at least one dimension and that it readily disperses in water as fairly stable colloids. However, filtration of water solubilized consumer products and personal care products indicated that less than 5% of the titanium was able to pass through 0.45 or 0.7 μm pores. Two white paints contained 110 μg Ti/mg while three sealants (i.e., prime coat paint) contained less titanium (25 to 40 μg Ti/mg). This research showed that, while many white-colored products contained titanium, it was not a prerequisite. Although several of these product classes contained low amounts of titanium, their widespread use and disposal down the drain and eventually to wastewater treatment plants (WWTPs) deserves attention. A Monte Carlo human exposure analysis to TiO2 through foods identified children as having the highest exposures because TiO2 content of sweets is higher than other food products and that a typical exposure for a US adult may be on the order of 1 mg Ti per kilogram body weight per day. Thus, because of the millions of tons of titanium-based white pigment used annually, testing should focus on food-grade TiO2 (E171) rather than that adopted in many environmental health and safety tests (i.e., P25), which is used in much lower amounts in products less likely to enter the environment (e.g., catalyst supports, photocatalytic coatings).
Provided by American Chemical Society