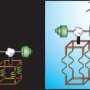
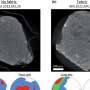
(Top left) A scanning electron microscope image of a copper-platinum nanobattery-based nanomotor. (Top right) A scanning electron microscope image of an asymmetric copper nanorod. (Bottom) Motion diagrams for each device in bromine solution. Image credit: Ran Liu and Ayusman Sen. ©2011 American Chemical Society
(PhysOrg.com) -- Measuring just 3.6 micrometers long, one of the smallest batteries ever made won’t be powering our electronic devices anytime soon, but it does serve as a self-powered nanomotor that is surprisingly fast and efficient. Ultimately, the nanobattery-based motor could be used as a nanomachine and to transport cargo for biomedical applications.
The researchers, Dr. Ran Liu and Prof. Ayusman Sen from the Department of Chemistry at The Pennsylvania State University, have published their study on the nanobattery-based motor in a recent issue of the Journal of the American Chemical Society ASAP.
The nanobattery consists of a single nanowire with a 3-micrometer-long copper end and a 600-nanometer-long platinum end. When the nanobattery is placed in a diluted solution of oxidant (such as bromine or iodine), the copper end serves as the anode and is oxidized while the platinum end functions as the cathode. As the nanobattery discharges itself in the solution, the electrophoresis phenomenon kicks in, so that the electric field generated by the battery’s redox reactions causes the battery to move.
Copper-platinum nanomotors move in an iodine solution (magnified 100x). The copper end of each nanomotor leads, while the brighter platinum end follows. Movement continues until the copper segments are completely converted to copper iodide by the iodine. Video credit: Ran Liu and Ayusman Sen. ©2011 ACS
“The scientific core of this finding is that a short-circuited nanobattery (e.g., copper-platinum segmented nanorod) can be moved by self-electrophoresis resulting from oxidation and reduction occurring, respectively, at the two metals,” Liu told PhysOrg.com. “The generated current can be directly converted to mechanical force.”
This self-electrophoresis phenomenon propels the device to speeds of more than 10 microns (three times its length) per second. That’s the rough equivalent of a 5-meter (16-foot) motorboat moving at 54 kilometers per hour (33.5 miles per hour) through water.
“In this case, the direction of the nanomotor’s movement is random at long time scales,” Liu said. “It can be potentially controlled. For example, if we incorporate a magnetic metal segment in the nanobattery, we can control its moving direction by magnetic field.”
The nanomotor operates continuously until the copper segment is completely oxidized by the bromine or converted to copper iodide by the iodine. Its lifetime, therefore, depends on both the length of the copper segment and the concentration of the oxidant. In their experiments, the researchers observed nanobattery lifetimes of between 40 seconds and 1 minute by changing these variables. (The length of the copper segment can be controlled by its electrodeposition time during fabrication.) The researchers found that the nanomotor’s velocity also depends on the length of the copper segment, where a shorter copper segment gives a higher speed but decreased lifetime.
Asymmetric copper nanorods undergo fast rotation in a bromine solution (magnified 50x). The rings of light around some nanorods are caused by the strong light reflection on the metallic surfaces. Video credit: Ran Liu and Ayusman Sen. ©2011 ACS
In addition, the researchers demonstrated that they could make the nanomotor function as a rotor out of copper only by polishing one side, causing it to be deformed into a “ratchet” shape. This asymmetric nanorod could rotate at extremely fast speeds of about 170 rpm in bromine. The scientists explained that the asymmetric shape generates a torque (or twist) that causes the rod to rotate.
The new nanomotor has some advantages over other self-powered nanomotors. For instance, the researchers previously built a gold-platinum nanomotor that used hydrogen peroxide as the fuel. However, this nanomotor produced oxygen bubbles that made it difficult to study and had a lower efficiency than the new copper-platinum nanomotor. The researchers attribute the new nanomotor’s improved efficiency to its electrolyte fuel, all or most of which is used to generate a current that is then directly converted into mechanical force. In contrast, most of the gold-platinum nanomotor’s fuel is wasted at the platinum end and not used to generate current.
“Our study confirms the generality of self-electrophoresis as a mechanism for micro/nanomotor movement and suggests that virtually any redox reaction occurring asymmetrically on an appropriate micro/nanostructure can be employed in the design of self-powered systems,” Liu said.
By demonstrating that self-electrophoresis of a nanobattery can provide propulsion to enable the nanobattery to function as a nanomotor, the researchers hope that the current results lead to future nanomotors with a similar design but that use different materials. For example, other metal pairs could be used, and different applications could be investigated.
“In principle, the nanomotors can be used to actively transport and deliver cargo, such as drugs, etc.,” Liu said. “In the future, we need to find more environmentally friendly, and especially biocompatible, fuel systems. Another remaining challenge is the design of moving micro/nanobatteries that can be recharged and used repeatedly.”
More information: Ran Liu and Ayusman Sen. “Autonomous Nanomotor Based on Copper-Platinum Segmented Nanobattery.” J. Am. Chem. Soc. DOI:10.1021/ja2082735
Copyright 2011 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.