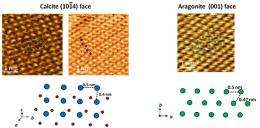
Atomic sequences of a calcite (1014) surface and an aragonite (001) surface along with their model drawings (bottom). Blue indicates the calcium atoms in calcite; red, the oxygen atoms; and green, the calcium atoms in aragonite. Credit: National Institute for Materials Science
Hard tissues of organisms, such as bones and shells, are composed of inorganic minerals (biominerals). While these substances are created by biomineralization, which will be discussed later, many uncertainties remain in the mechanism.
Research scientists at Tohoku University, Japan, have been conducting studies designed to elucidate the crystal growth mechanisms of shells, in which a calcium carbonate crystal, which is normally stable only in a high-pressure phase, is formed under normal temperature and pressure conditions. It is necessary to observe the atomic structural changes of the growing crystal surface in solution. Under such circumstances, a system capable of high-resolution surface observation in a liquid environment would be ideal.
Changes in the atomic sequence of the calcite substrate surface with an added growth solution containing the synthetic polypeptide and magnesium. The period required for one scan in this case was approximately 8 seconds. Credit: National Institute for Materials Science
Tsukamoto and Araki learned that the atomic force microscope (AFM) probe system developed by the Yamada group at Kyoto University could operate under such conditions and was available for use via the Kyoto Advanced Nanotechnology Network; an application was made for joint utilization and support. By using the FM-AFM system, based on leading-edge frequency-modulation atomic force microscope (FM-AFM) technology, they successfully observed the calcium carbonate crystal growth process in solution on the atomic level.
More information: nanonet.nims.go.jp/english/magazine/?Vol.%204%2C%20No.%204%2C%202011-08-24%2FFocus%2026-18
Provided by National Institute for Materials Science